Ultimate Resource For Covid19
FDA Says Pfizer-BioNTech Vaccine Is Safe, Effective. Updated: 2-25-2021: Novavax Nears Covid-19 Vaccine Game Changer—After Years of Failure. Ultimate Resource For Covid19

In January of last year, employees of Novavax Inc. met at a local Maryland bar to discuss how they might salvage their careers.
For decades, the small biotech had tried to develop an approved vaccine, with no success. The company had enough cash to survive only another six months or so and its shares traded under $4, with a market value of $127 million.
Related:
Ultimate Resource On Long COVID or (PASC)
Harvard Chemical Biology Department Chair Accused Of Selling Covid19 To Wuhan University
Paying Off Unfunded Pension Liabilities Will Be A Low Priority After COVID-19
US Says China Backed Hackers Who Targeted COVID-19 Vaccine Research
Ultimate Resource For Covid-19 Vaccine Passports
Companies Plan Firings For Anti-Vaxers And Giveaways For Covid-19 Vaccine Recipients
Four Stories Of How People Traveled During Covid
How To Travel Luxuriously Post- Covid-19, From Private Jets To Hotel Buyouts
‘I Cry Every Day’: Olympic Athletes Slam Food, COVID Tests And Conditions In Beijing
Lessons Of The Great Depression: Preserving Wealth Amid The Covid-19 Crisis
Cyber Attack Hits Health And Human Services Department Amid Covid-19 Outbreak
Ultimate Travel Resource Covering (Covid-19, Business, Personal, Cruise, Flying, Etc.)
Today, Novavax is advancing toward authorization of a Covid-19 vaccine. Scientists believe that, if cleared, it could be one of the more powerful weapons against the pandemic, offering key possible advantages over its competitors.
Some early data suggest the Novavax shot may be one of the first shown to stem asymptomatic spread of the coronavirus and also potentially provide longer-lasting protection.
If the two-shot regimen is authorized, Novavax will still face the challenge of making and distributing it in large quantities. The firm sold some manufacturing assets in 2019 when it was desperate for cash.
Investors, who left the 33-year-old company for dead last year, are betting that regulators will authorize Novavax’s vaccine in the next couple of months. They have sent shares on Nasdaq up to $229, up 106% so far this year.
Late last month, Novavax released preliminary data indicating its shot was effective at protecting against Covid-19, though less so against a new strain identified in South Africa that appears to be a challenge for other shots, too. Results of the vaccine’s late-stage U.S. trial could be released late next month.
Novavax now has a market value of $15.4 billion, greater than that of companies with billions of dollars of annual sales, including generic drug giant Teva Pharmaceutical Industries Ltd.
An authorization would reinforce just how much Covid has upended the pharmaceutical world. The most advanced vaccines have been introduced by newcomers to the vaccine market using previously unproven technologies that can be designed and manufactured more quickly than conventional options.
While vaccine giant Merck & Co. recently shut down its Covid-19 vaccine effort after disappointing results, upstarts like Moderna Inc. and BioNTech SE quickly developed, tested and manufactured shots currently going into arms around the world.
Novavax’s vaccine “looks as strong as any vaccine, and it may have better durability,” says John Moore, an immunologist at Weill Cornell Medical College in New York. Initially skeptical of Novavax and its shot, Dr. Moore said he grew so encouraged by early study data last year that he volunteered to be a study subject and purchased some shares in the company, which he has since sold.
Much is riding on Novavax’s success. Rollouts of shots from Pfizer Inc. and partner BioNTech, Moderna, and AstraZeneca PLC have been slower than anticipated. The companies have struggled to meet huge demand.
Novavax says it could produce a couple billion doses over the next year, beginning around April, equal to or more than those expected from other companies.
Last week, Novavax announced a deal with Geneva-based public-health institution GAVI to allocate doses around the globe.
“I admire their tenacity, their persistence, their never-say-die approach,” says Roger Pomerantz, an infectious-disease and vaccine veteran who is chief executive of biotech ContraFect Corp. and a former executive at Merck, which several years ago passed on working with Novavax. “But at the end of the day, you need to see vaccines.”
Unlike shots from Pfizer and Moderna, the two authorized in the U.S., Novavax’s vaccine doesn’t need to be kept at freezing temperatures. That’s an advantage for hospitals, clinics and pharmacies lacking freezer capacity.
While Novavax has developed some proprietary methods, its vaccine approach is similar to those of proven vaccines, such as those for shingles and hepatitis B—one reason why some scientists are fans.
The Pfizer and Moderna shots are based on a newer technology that delivers genetic code through molecules known as messenger RNA, instructing the body to create the coronavirus’s signature spike protein, which elicits an immune response.
AstraZeneca’s vaccine, based on a different technology and authorized for use in Europe and the U.K., delivers a harmless chimpanzee virus carrying similar directions.
Novavax’s vaccine injects a synthesized, slightly modified version of the spike protein, rather than instructing the body to create it. The company’s scientists first take a common insect virus, called a baculovirus, and insert DNA instructions to create the spike protein. They use the newly infused baculovirus to infect cells that originally came from an armyworm insect.
The cells are grown in large bioreactors holding up to 6,000 liters of liquid—vats that look like they come from breweries—where the spike protein is produced. The protein is isolated, purified and injected into people as a vaccine, along with a compound, called an adjuvant, that jump-starts an immune response and renders the vaccine more effective.
Novavax’s bumpy history shadows its efforts. Sixteen years ago, its lead product was a cream for postmenopausal women dealing with hot flashes. It wasn’t selling. Facing bankruptcy, the company turned to Gale Smith, now 71, a molecular biologist who was running its small vaccine group.
As a graduate student, Dr. Smith had helped invent the approach of using baculoviruses to shuttle the DNA of an infectious agent into insect cells to create vaccines, a method that today is used for a flu vaccine and another for human papillomavirus, or HPV.
For the next decade or so, the company employed Dr. Smith’s approach to develop vaccines for HIV, SARS, swine flu, Ebola, Middle East respiratory syndrome and more. Each time, Novavax achieved promising early results and gained backing from government bodies and nonprofits, including the Gates Foundation.
Ultimately, either the shots failed in further testing or the epidemics ebbed, reducing the need for a vaccine.
Executives tried to lift staffers’ spirits amid the disappointments. Most Fridays, Chief Executive Stanley Erck, a 72-year-old Vietnam War veteran, led employees to a nearby bowling alley, where they competed over pizza and beer. Gregory Glenn, a pediatrician who runs research and development, tried to persuade employees to stick around.
“Where else can you do something noble, interesting and get paid for it?” he says he told researchers.
Senior executives and others began quitting, weighing on Dr. Glenn. Driving home one night a few years ago, he broke down crying, he says. “I felt I let them down and had been overly enthusiastic,” he says.
In 2014, Novavax purchased a Swedish company that produced an adjuvant, derived from the bark of a Chilean tree, to boost the immune response generated by a vaccine.
Using the adjuvant, Novavax began working on shots to protect against respiratory syncytial virus, or RSV, which causes the hospitalization of about 58,000 young children a year in the U.S. and can be deadly.
In 2015, the stock doubled as Mr. Erck said the vaccine had the potential to be “the largest selling vaccine in the history of vaccines in terms of revenue.”
“I’m a biotech CEO, I admit to being an optimist,” Mr. Erck says.
The next year, the vaccine failed in late-stage, or Phase 3, trials. The morning after the 2016 presidential election, about one-third of staffers were greeted by messages they were being laid off, due to the RSV failure.
“It was a huge surprise, a sad and gloomy day. People were crushed,” says Kwanho Roh, a cell biologist who survived the layoffs but saw a third of his team let go before he left the company.
By 2019, after another RSV trial failed, Novavax had cemented a reputation as the little company that couldn’t. When Mr. Erck spoke at industry conferences, sometimes as few as three people came to hear him, he says.
In May of that year, with the stock at 36 cents a share and in danger of getting tossed off the Nasdaq market, Novavax did a 1-for-20 reverse stock split. It finished 2019 down another 39%.
In late 2019, Nasdaq removed Novavax’s shares from its biotechnology index, but Dr. Smith was sure his team was making further progress tweaking their vaccine.
As 2020 began, Novavax was conducting late-stage trials on yet another vaccine, this time for flu. The company was also exploring options to advance its RSV vaccine, which has shown efficacy, including holding discussions with regulators and potential commercial partners.
Early data on the flu vaccine was impressive but staffers fretted about the dwindling cash. Mr. Erck knew it was his company’s final chance, and that some employees already had one foot out the door.
When the pandemic hit, executives made a difficult decision to shift to a Covid-19 vaccine, risking delays in their flu efforts. They had done work on earlier coronaviruses, so they figured they had a chance at success.
In January, Novavax ordered the gene for the spike protein from a supplier with an office in Shanghai, but it never came—flights from China had been frozen. After a few days, the supplier’s New Jersey office agreed to produce the gene and drive the red-capped vial overnight to Novavax’s offices outside Washington, D.C.
Weeks later, they used their baculovirus method to create a vaccine that needed to be tested in trials. However, Novavax didn’t have the cash to make the shots in bulk.
In March, Mr. Erck joined other pharmaceutical executives in a meeting at the White House to discuss Covid-19 vaccines. He was blunt about Novavax’s challenges. “Frankly, we need money,” he told President Trump, according to a transcript of the conversation.
Novavax lined up about $2 billion in combined funding from Operation Warp Speed, the U.S. government’s initiative to speed development of Covid-19 vaccines, and the Coalition for Epidemic Preparedness Innovations, a Norway-based nonprofit. Novavax raised additional money from a big hedge fund.
To test the vaccine, Dr. Glenn called researchers at the University of Oklahoma, who offered 15 baboons bred for medical research. The subsequent trial, along with other animal studies and early human trials, demonstrated enough efficacy to stir buzz in the industry.
In October, the company pushed back the start of its late-stage U.S. trial, falling further behind rivals, due to the complexities of manufacturing the shots.
On Jan. 28, Novavax said an interim analysis from a Phase 3 study showed its vaccine was 89% effective at protecting people from the disease in the U.K., where a worrisome strain of the coronavirus has been circulating.
The vaccine was just 49% effective overall in a separate, middle-stage study in South Africa, where another variant is spreading, though the figure was 60% among those who weren’t HIV-positive.
Like other Covid-19 vaccine makers, Novavax is reformulating its shots to better handle the new virus strains.
Larry Corey, an infectious-disease specialist at the Fred Hutchinson Cancer Research Center who helped set up the federally funded trials of several of the Covid-19 vaccines, says Novavax’s vaccine has several potential advantages, including a relatively low dose level that should support mass production and enable supplies to be stretched out.
“Its global applicability and potential are very high,” Dr. Corey says.
Analysts at Sanford C. Bernstein say if Novavax’s is cleared, it could generate revenues of $5 billion this year. But Novavax executives, more aware than most that vaccines often fail in late trials, aren’t ready to celebrate.
Investors have bet against nearly 10% of Novavax shares, a sign of their lingering doubts. By comparison, stocks in the S&P 500 have an average short-rate—a measure of shares sold short as a percentage of those outstanding—of 2.5%.
Results of the large U.S.-based trial could be available by late March. Mr. Erck says he is nervous but optimistic. Others close to him remain on edge. His wife, Dr. Sarah Frech, has recently been rubbing a favorite doll, something she does to bring good luck.
“She’s more superstitious than I am,” he says.
Updated: 1-29-2021
J&J Vaccine Provides Strong Shield Against Severe Covid
Johnson & Johnson’s one-shot vaccine generated strong protection against Covid-19 in a large, late-stage trial, raising hopes that it can rapidly reshape a stumbling immunization campaign.
In a study of more than 43,000 people, the vaccine prevented 66% of moderate to severe cases of Covid-19, according to a company statement Friday. And it was particularly effective at stopping severe disease, preventing 85% of severe infections and 100% of hospitalizations and deaths.
“If you can prevent severe disease in a high percentage of individuals, that will alleviate so much of the stress and human suffering” of the pandemic, said Anthony Fauci, the top U.S. infectious-disease official, at a briefing on the results with company and government officials.
Based on the result, J&J plans to file with the U.S. Food and Drug Administration for an emergency-use authorization next week. The drug giant’s top scientist said this month he expects a clearance in March, and that it would have product ready to ship then.
The company didn’t specify how much of the vaccine would be available immediately, though it reaffirmed that the U.S. would receive 100 million doses by the end of June.
If cleared, J&J’s vaccine could go a long way toward ending the pandemic. Competing vaccines from Moderna Inc. and Pfizer Inc. have generated stronger overall efficacy rates of more than 90%, but require two shots to be given before their full benefits take hold. J&J’s shot also can be kept in a refrigerator for three months, while those from Pfizer and Moderna must be kept frozen.
“This is a single shot that can be given easily, it protects completely from that which we fear, having to go to the emergency room or a hospital,” said Mathai Mammen, head of global research and development for J&J’s pharmaceutical division, in an interview. “It’s going to change the nature of the disease.”
White House Press Secretary Jen Psaki said in a briefing Friday that President Joe Biden is encouraged by the “positive data” from J&J and believes that “now is the time for the FDA to do its job evaluating the safety and efficacy of the vaccine.”
Variants Abound
Concerns about keeping second doses of the current shots on hand have complicated the push to get as many people as possible inoculated. Some countries have chosen to spread out the time between doses to address the problem, at the risk of diminishing their effectiveness.
The rise of new coronavirus variants has added to the pressure to get immunizations moving faster. The J&J trial was conducted around the globe, including at dozens of clinical-trial sites in hot spots such as South Africa and Brazil where new variants have caused infections to rise.
J&J’s results produced more evidence that the variants will be harder to ward off. In the U.S., where mutations aren’t thought to be as widespread, the vaccine was 72% effective.
But in South Africa, where a variant called B.1.351 is circulating widely, it was only 57% effective. And the shot was 66% effective in Latin America.
Nonetheless, J&J’s vaccine is likely to give countries around the world a powerful new tool to fight a virus that has infected more than 101 million people and killed 2.2 million worldwide.
If confirmed, the results suggest people could get one dose of the vaccine to provide initial protection against severe consequences, allowing them to return to their pre-pandemic lives.
Then, if needed, they could later take a booster shot J&J is testing in other large, late-stage trials that could produce results before year-end.
Protection was consistent across race, age groups, including those over the age of 60, and regions. J&J’s Mammen added that the shot appears to generate immunity that ramps up and sustains itself over time.
The drug giant is also studying the shots’ ability to prevent asymptomatic infections, and will soon report data to that end, Mammen said.
J&J said a review by a monitoring board identified no significant safety concerns. While 9% of people who took the shot developed a fever, there were no severe allergic reactions.
Different Technology
J&J’s vaccine is different from the messenger RNA-based shots made by Moderna and partners Pfizer and BioNTech SE.
It is based on an adenovirus, or cold germ modified to make copies of the coronavirus spike protein, which the pathogen uses to force its way into cells.
The altered virus can’t replicate in humans, but it triggers an immune response that prepares the body to defend itself against the coronavirus. J&J uses the same technology in a vaccine to fight Ebola.
J&J’s R&D head said the company’s trial, conducted at the height of the pandemic, had to deal with resistant variants that arose mainly after Moderna’s and Pfizer’s trials were finished. When counting cases, it also focused on somewhat sicker patients, Mammen said.
“If those vaccine programs accrued cases at the same time as us, when viral infections were so much higher, incidents were higher, and variants were all around us, they would have gotten different numbers,” he said. “The fact that we could do this level of efficacy with a single shot, people don’t have to come back for another, and it’s conveniently stored, well that makes this the vaccine of choice.”
At the outset of the pandemic, U.S. government officials said any vaccine showing greater than 50% efficacy would be considered a success.
Like Pfizer and Moderna, Mammen said J&J is working on next-generation versions of the shot that could provide protection specifically against certain variants. Other vaccines have seen mixed results against the newer forms of the virus.
On Thursday, Novavax Inc. reported that a large late-stage study in the U.K. found its Covid vaccine was 89% effective.
However, it was just 60% effective in South Africa in people who were HIV negative, and 49.4% effective when HIV positive patients were included. Most cases of the virus seen in the trial had the new South Africa mutation, the company said.
J&J “is already testing and creating vaccines with the ability to respond quickly to the South African strain,” Mammen said.
Fauci said Friday that the U.S. and its pharmaceutical partners must be active in responding to current and future variants.
“It’s a wake-up call for us to be nimble and to be able to adjust, as this virus will continue, for certain, to evolve and mutate,” he said.
Gaining Clearance
J&J aims to have a total of seven manufacturing facilities running by the end of the second quarter, Chief Financial Officer Joseph Wolk said Jan. 26. The company says it remains on track to hit the goal of producing 1 billion doses globally before year-end.
J&J’s candidate was among six vaccines tapped for the Trump administration’s Operation Warp Speed program, receiving some $1.5 billion in backing from the U.S. government. The company intends to price the vaccine on a not-for-profit basis that won’t exceed $10 a shot, Wolk said.
Fauci said the scalability and low cost of the shot make it a “value-add” for the U.S. government’s vaccine portfolio.
The Centers for Disease Control and Prevention is reading Johnson & Johnson’s data “as quickly as we can,” said John Brooks, chief medical officer, CDC Covid-19 response, on a call with the Infectious Diseases Society of America.
“Any vaccine is better than no vaccine” as long as it meets FDA standards for emergency-use authorization, Brooks said. He added that he would encourage anyone offered a vaccine that has received an emergency authorization to take it.
While the trial readout marks the start of a new phase in the pandemic response, it also is the close of an anxious chapter in the lives of those who developed it. Mammen said he was in his basement office in his home in New Hope, Pennsylvania, when he learned the results.
“I’ve been at this for about a year, every day, morning through night,” he said. “When it was unblinded, there was this massive, I mean massive, sense of relief. And great joy. I could finally sleep.”
Updated: 1-8-2021
Pfizer, BioNTech Shot May Defeat New Variants, Study Shows
Pfizer Inc. and BioNTech SE’s Covid-19 vaccine may protect against the new fast-spreading variants of the coronavirus that have emerged in the U.K. and South Africa, according to a study.
The study, by researchers at the University of Texas Medical Branch and supported by the companies, homed in on the crucial N501Y mutation in the virus’s spike protein that is common to both fast-spreading variants.
Antibodies in the blood of people who had been vaccinated were able to neutralize a lab-created version of the mutant virus.
Though it’s early data, the results are a promising sign that the vaccine will probably have an effect against the new variants, a major worry for health authorities that are struggling to stem a tide of new infections even as they seek to vaccinate hundreds of thousands of vulnerable people. Both the U.K. and South African strains appear to be more infectious than previous mutations.
The research examined the response to the mutant viruses in blood samples taken from 20 people who had gotten the companies’ mRNA vaccine as part of a previous clinical trial.
The research didn’t study other mutations in the spike protein. Still, the antibodies in the vaccinated people’s blood did just as good a job at disarming the mutant virus as they did with the non-mutant version.
“This is clearly a positive, but there are important caveats to add,” Adam Barker, a London-based analyst with Shore Capital Group Ltd., wrote in a note.
The study only addresses one mutation and doesn’t show whether the vaccine can actually prevent people from being infected with the new variant, he wrote. “That being said, the working assumption remains that vaccines will be at least partly effective against the novel variants.”
The findings were also consistent with the results of tests on 15 other mutations that have been found in SARS-CoV-2 strains, the researchers said.
“We are encouraged” by the early findings, Pfizer Chief Executive Officer Albert Bourla said in a tweet.
We are encouraged by early in vitro study findings that show the sera from people who have received the Pfizer-BioNTech #COVID19 vaccine effectively neutralized a SARS-CoV-2 virus having one of the mutations also found in two highly transmissible strains. https://t.co/y5BtV6gQjm https://t.co/nbdzwFlHSq
— AlbertBourla (@AlbertBourla) January 8, 2021
Executives at BioNTech — as well as from Moderna Inc., the developer of a rival mRNA shot — had previously said they believed their vaccines would protect against the new strains.
The University of Texas study is one of the first to back up those claims. The results were released on the bioRxiv preprint server early Friday, ahead of peer review.
The research comes as Covid-19 spreads globally at record daily levels, likely accelerated by the new strains, and as countries begin to roll out vaccines.
The U.K. variant, which has been identified across the U.S. as well as in countries from South Korea to Canada, is thought to be 57% to 70% more transmissible than other strains of the virus.
Viruses have the opportunity to change through mutations that arise naturally as they replicate and circulate in their hosts. Some, like influenza, evolve quickly with thousands of mutations and distinct lineages, while others are more stable.
Updated: 12-29-2020
CDC Advisory Panel Votes To Recommend Moderna’s Covid-19 Vaccine
A Centers for Disease Control and Prevention advisory panel voted Saturday to recommend Moderna Inc.’s coronavirus shot for people 18 and older, paving the way for the second Covid-19 vaccine to be administered in the U.S.
Members of the CDC’s Advisory Committee on Immunization Practices voted 11-0 to recommend the shot, with three abstaining because of conflicts of interest.
The advisers cast the vote in an emergency meeting Saturday after the Food and Drug Administration authorized use of Moderna’s shot Friday.
The recommendations are key to helping vaccine providers understand best practices for administering the vaccine. CDC Director Robert Redfield must sign off on the group’s decisions before they are made final. States, a handful of large cities and territories are slated to soon receive some of the 5.9 million doses of Moderna’s vaccine that the federal government plans to initially release.
Moderna’s vaccine, the second to be authorized for emergency use in the U.S. this month, will be introduced amid more than 200,000 new daily coronavirus cases being reported and record hospitalizations.
“I was very eager to put forth this nomination for a second vaccine that could be lifesaving, especially in light of the fact that we are seeing an average 2,600 deaths a day,” said Lynn Bahta, committee member and immunization specialist at the Minnesota Department of Health. “This is horrendous.”
Peter Szilagyi, a pediatrician at the University of California, Los Angeles and CDC advisory panel member, said the benefits of Moderna’s Covid-19 vaccine far outweighed any risks given the pandemic.
The CDC immunization panel process has been “rigorous, fair and transparent,” Szilagyi said, adding that he was impressed with the CDC’s “V-safe” system for enabling people to log any symptoms experienced after receiving Covid-19 vaccines to ensure real-time tracking of adverse events.
Allergic Reactions
A handful of serious allergic reactions were reported in the U.K. and the U.S. from Pfizer Inc. and BioNTech SE’s Covid-19 vaccine. Moderna’s formula includes similar components, prompting committee members to weigh how to monitor people for such reactions.
In the U.S., the CDC has identified six cases of anaphylaxis among 272,001 doses of Pfizer-BioNTech’s shot administered as of Saturday morning, the CDC’s Tom Clark told the committee.
The six people were under the age of 65, Clark said. There is no obvious geographical clustering of cases despite reports of multiple instances of anaphylaxis occurred at some hospitals, Clark said.
The CDC recommends vaccine providers monitor most patients for 15 minutes and those with a known history of anaphylaxis for 30 minutes. Vaccine administration sites should stock medications such as epinephrine, according to proposed information for clinicians presented during the meeting.
Pharmacists and doctors administering Pfizer-BioNTech’s Covid-19 vaccine recognized some vials contained enough liquid to give one or two more shots than they expected. The same could happen with Moderna’s shot, though the company expects that won’t happen often, a company representative told the committee.
Updated: 12-28-2020
Highly Touted Monoclonal Antibody Therapies Sit Unused In Hospitals
Medical centers overrun with critical Covid-19 patients are using just a fraction of allotted supplies of the treatment.
Doses of monoclonal antibodies—Covid-19 therapies authorized for emergency use last month—are sitting unused in hospital pharmacies, even as cases surge across the country.
Hospitals say the rollout of the therapies has been stunted by a lukewarm response from infectious-disease specialists, who say they want more clinical trial data before using them on a regular basis.
Medical centers are also grappling with a lack of awareness and interest from both the primary-care doctors who would normally prescribe the drug and patients who are offered it. And some places are dealing with a shortage of space and staff to administer the therapies.
When monoclonal antibody therapies from Eli Lilly LLY -0.10% & Co. and Regeneron Pharmaceuticals Inc. were approved for emergency use in November, health agencies were worried there wouldn’t be enough supply to meet demand.
Now, health-care providers are administering just 20% of the doses they receive each week, according to officials with Operation Warp Speed, the federal initiative to support development of new drugs, vaccines and diagnostics for Covid-19.
Monoclonal antibodies, which are also used to treat other diseases, work by taking a page from the body’s own natural antibody defenses, targeting specific spots on intruding pathogens.
Eli Lilly’s bamlanivimab and Regeneron’s casirivimab-and-imdevimab cocktail target the SARS-CoV-2 spike protein and are injected intravenously.
Early trial data of the therapies found they could reduce hospitalization or emergency visits among high-risk patients.
“Physicians are not ordering the drug,” said Michael Ison, an infectious-disease physician at Northwestern Memorial Hospital who is helping lead the monoclonal-antibody rollout there and in the wider Northwestern Medicine health system, which includes 10 hospitals across the Chicago area and northern Illinois.
Demand from doctors in the Northwestern system has been relatively low and many patients aren’t all that interested, he said. Although several hospitals have set up spaces for the infusions and have made arrangements for staff to deliver them, some physicians just aren’t comfortable with prescribing the therapy because it is so new and it is hard to discern which patients will benefit from it, Dr. Ison said.
The monoclonal antibody treatments from Eli Lilly and Regeneron were approved by the U.S. Food and Drug Administration for use in patients with mild or moderate Covid-19 who are at high risk of progressing to severe symptoms or hospitalization.
The FDA has a specific definition of what high risk means, which includes people who are 65 years of age or older, or people who are considered obese, with a body-mass index of 35 or more.
The therapies are supposed to be administered as soon as possible after a patient receives a positive test result, within 10 days of the onset of symptoms.
But sussing out who would benefit and when isn’t easy, doctors say. And patients sometimes recover on their own within a couple of days, which renders the therapy moot. Often, patients with mild symptoms refuse the therapy after being offered it, doctors say.
“A significant number of patients have declined it,” said Emily Rubin, a pulmonary and critical-care physician who has been helping lead the monoclonal antibody rollout at Massachusetts General Hospital in Boston.
MGH has received about 275 doses of Eli Lilly’s bamlanivimab, but only 10% of that has been administered so far, she said. The hospital also received a small supply of Regeneron’s product, but that hasn’t been used at all, she said.
Some patients who meet the high-risk criteria have mild or moderate symptoms that are improving, so they aren’t interested in coming in for an infusion, Dr. Rubin noted. Others aren’t able to spare the two hours required for administration and monitoring. Still others are told of the benefits of the drug according to trial data and decide the benefit is too dubious.
A recently published interim analysis of Regeneron’s cocktail found that it could reduce viral load in some patients, and an interim analysis of bamlanivimab found that five out of 309 patients who received the therapy required a visit to the emergency department or hospitalization, compared with nine out of 143 of the placebo population.
But some infectious-disease physicians aren’t convinced, saying more data are needed.
“To be really certain about the results, you need greater numbers,” said Rajesh Gandhi, an infectious-disease physician at MGH and a member of the Covid-19 treatment guidelines panels at the National Institutes of Health and the Infectious Diseases Society of America.
The NIH has said there are insufficient data to recommend for or against the use of the Eli Lilly and Regeneron treatments. Neither should be considered the standard of care for treatment of patients with Covid-19, NIH said. The IDSA also recommends against the routine use of bamlanivimab.
The need for more data doesn’t mean people shouldn’t be treated now, said Rich O’Neal, Regeneron’s vice president of market access. “It’s going to be really challenging if we continue to wait too long for information and data to continue to decrease the impact of the crisis.”
“The emergency-use authorization is based on a standard of data which is different than the normal drug approval.
We fully acknowledge that,” said Daniel Skovronsky, Eli Lilly’s chief scientific officer. “On the other hand, we’re excited about the potential that we’ve seen in our clinical trials: particularly, we saw reduced hospitalizations and emergency-room visits. Although it was a small trial, Regeneron had almost the exact same kind of impact—a different molecule but the same mechanism.”
UW Medicine, which has four hospitals, largely isn’t using the monoclonal antibody therapies, said Shireesha Dhanireddy, an infectious-disease doctor at the Seattle-based system.
Although the hospital system has made a few limited requests for some of Washington state’s supply of Eli Lilly’s monoclonal antibody drug bamlanivimab, UW Medicine isn’t routinely using it, she said, after deciding the benefits were too uncertain and administering the drugs would add additional strain on health-care workers.
Administering bamlanivimab is time-intensive, she said, and staff have been urgently needed to care for rising numbers of Covid-19 patients.
UW Medicine in late November and early December also needed staff to complete plans to vaccinate its employees for the novel coronavirus, an effort that is now under way, Dr. Dhanireddy said.
In contrast, health-care system Northwell Health in New York is moving ahead with its monoclonal-antibody rollout, setting up five sites where it can administer the intravenous drugs.
Some are located in tents that were used as overflow units during the Covid-19 surge in New York City in the spring, said Warren Licht, vice president of ambulatory operations at Northwell who has been leading the effort. One is located in the emergency room of Northwell’s Cohen Children’s Medical Center, he said.
He said Northwell hopes to open more sites and is planning for more emergency-room locations. He is also hoping to include Northwell’s skilled-nursing facilities in the rollout and to set up a program for home infusions.
States are beginning to expand the availability of infusions outside of the hospital, including in nursing homes and outpatient infusion clinics, and offering to administer the drugs in patients’ homes, said Janet Woodcock, head of drug evaluation and research at the FDA who is on leave to work with Operation Warp Speed.
In the future, doctors’ offices could also start offering to provide the treatment, which may be particularly helpful in rural areas where there aren’t many nearby health facilities, she said.
Updated: 12-15-2020
FDA Finds Moderna Covid-19 Vaccine Highly Effective
Second major U.S. coronavirus vaccine could possibly be shipped by this weekend.
The Food and Drug Administration said Tuesday that the Covid-19 vaccine developed by Moderna Inc. MRNA -5.06% was “highly effective,” setting the stage for an emergency authorization later this week that would add a second vaccine to the arsenal against the pandemic.
The agency posted online documents, prepared by its staff and by Moderna, analyzing the safety and effectiveness of the vaccine in a large clinical study. The findings will go before an independent advisory panel that will vote Thursday on whether to recommend FDA authorization.
Barring complications, the FDA is aiming to authorize emergency use of the Moderna vaccine Friday, following the same timetable as last week with the first Covid-19 vaccine from Pfizer Inc. and BioNTech SE.
Moderna’s analysis, posted by the FDA, also included new data suggesting that the first dose of its vaccine can reduce infections that don’t cause symptoms. If this finding holds up in further analysis—including after the second of the two-dose regimen—it could mean that the vaccine not only protects individuals from disease, but also curbs transmission of the virus from person to person.
A vaccine that prevents asymptomatic infections and curbs viral transmission could hasten the end of the coronavirus pandemic, if enough people get vaccinated.
“If we could demonstrate that they reduce transmission, that would accelerate the time when we can take off our masks and go back to a more normal semblance of life,” said William Schaffner, professor of health policy and preventive medicine at Vanderbilt University. He said more data are necessary to conclude whether the Covid-19 vaccines can do that.
A Pfizer researcher said last week the company was studying whether its vaccine protects against asymptomatic Covid-19 and hopes to complete that analysis early next year.
Doses of Moderna’s vaccine could be shipped this weekend, with vaccinations starting early next week. Which vaccine people get will be decided by factors including availability, with the Centers for Disease Control and Prevention and the Trump administration’s Operation Warp Speed overseeing distribution.
Federal officials have said the Moderna vaccine will be more suitable for smaller hospitals in rural areas because it is shipped in smaller quantities than Pfizer’s.
The FDA’s review of a 30,000-person clinical study confirmed Moderna’s earlier disclosure that the vaccine was 94.1% effective at preventing Covid-19 disease with certain symptoms, including severe disease.
The agency also said there were no specific safety concerns that would preclude authorization, though new details in the documents show that people can experience significant side effects from the shot.
The Moderna vaccine, if authorized, would be the first of several expected to augment U.S. vaccine supplies following the rollout to Americans this week of the first shot, from Pfizer.
Vaccines are considered critical to ending the pandemic, which has killed about 300,000 people in the U.S. and more than 1.6 million world-wide.
FDA analysts found that the Moderna vaccine was effective “across age groups, genders, racial and ethnic groups, and participants with medical comorbidities [underlying conditions] associated with high risk of severe Covid-19.”
Similarly encouraging, FDA scientists also found that the research “suggested benefit of the vaccine in preventing severe Covid-19.” The issue of effectiveness against severe disease has been raised about the studies of Covid-19 vaccines.
The study found 30 cases of severe disease in the placebo group, versus zero in the vaccine group.
Moderna found that some people who started the study with no evidence of infection developed asymptomatic infections after the first dose and before the second dose.
Of these, Moderna found there were about two-thirds fewer asymptomatic coronavirus infections among vaccine recipients than among those who had gotten a placebo, according to a Moderna document posted by FDA. The document didn’t include results following the second dose.
Moderna’s new data showing a reduction in asymptomatic infections after the first dose means it is possible to “slow down the spread of the virus in the community by being vaccinated, in addition to protecting yourself from severe disease,” said Moderna Chief Executive Stephane Bancel.
Moderna previously has said its experimental vaccine was 94.1% effective in protecting people against Covid-19. In that first public, full analysis of the pivotal study, the Cambridge, Mass., company said that 196 people in the 30,000-person trial experienced symptoms and that 185 of those were given a placebo, versus just 11 on the Moderna vaccine.
The FDA analysis found the Moderna vaccine appeared somewhat more effective in younger people than in seniors.
Vaccine efficacy was 95.6% among people 18 to 64, and 86.4% among those 65 and older.
Moderna studied its vaccine in people 18 and older, and is seeking authorization for use in that population. The FDA cleared Pfizer’s vaccine in people 16 and older because the study included that age group.
The FDA found no specific safety concerns that would preclude its authorization of the vaccine. The most common side effects included injection-site pain, fatigue, headache and chills. Severe adverse reactions were rare but occurred more frequently after the second dose than after the first dose.
For some people, these side effects can be significant. For instance, about 9.1% of vaccine recipients had injection-site reactions that were classified as “grade 3,” which the FDA defines as severe or medically significant but not immediately life threatening. In comparison, fewer than 1% of placebo recipients had grade 3 injection-site reactions.
Some 16.5% of vaccine recipients had systemic adverse reactions, such as fever and fatigue, with a severity of at least grade 3, versus 3.7% among placebo recipients. Severe fatigue was more common after the second dose than after the first dose.
The FDA also found a higher rate of hypersensitivity events among vaccine recipients than those who received placebo. There were no anaphylactic or severe hypersensitivity reactions closely related to the vaccine in the study, the FDA said.
In the U.K., two health-care workers had severe allergic reactions to the vaccine from Pfizer and BioNTech, prompting regulators there to caution against vaccination for people with a history of allergic reactions.
The FDA said it would recommend that vaccinated people be monitored for a condition called Bell’s palsy, which weakens facial muscles, because three vaccine recipients in the Moderna study experienced it. This condition also was reported in a small number of recipients of the Pfizer vaccine in the Pfizer study.
The FDA’s Vaccines and Related Biological Products Advisory Committee will discuss the Moderna data Thursday. The same panel reviewed the Pfizer data at an all-day meeting last week, after which it voted 17-4, with one abstention, in favor of granting emergency-use authorization.
The FDA did so one day later and is expected to move quickly to approve the Moderna vaccine if there is a favorable recommendation.
The effectiveness of Moderna’s vaccine essentially duplicated that of Pfizer’s vaccine, which had 95% efficacy. The first U.S. vaccinations using Pfizer’s shot outside of clinical trials began Monday.
Moderna’s novel technology, like Pfizer and BioNTech’s, employs messenger RNA, a naturally occurring molecular courier. In essence, it instructs human cells to make a spike protein of the sort found on the coronavirus’s cells to trick the body into mounting an immune response.
Use of that technology so far appears to slash the traditional production time for vaccines, which have tended to take a decade or more to develop.
While the vaccines shared many similarities, they were studied somewhat differently.
For the main measure of effectiveness in the Pfizer trial, researchers started keeping count of Covid-19 cases among volunteers one week after they received the second dose of either the vaccine or a placebo. In Moderna’s trial, researchers started counting cases two weeks after the second dose.
Also, the studies had different criteria for the severity of the Covid-19 cases prevented by the respective vaccines. In Pfizer’s trial, it was a positive Covid-19 test plus at least one symptom such as fever, cough or chills.
In Moderna’s trial, it was a positive test, plus at least two systemic symptoms such as fever and chills, or at least one respiratory symptom such as cough or shortness of breath.
Moderna has said it expects to have 20 million doses available for the U.S. to ship by the end of 2020, enough to inoculate 10 million people with the two-shot regimen. The company tested the vaccine in people 18 and older, comparing them with subjects who got a placebo injection.
Moderna, with its own manufacturing plant and working with contract manufacturers, expects to produce between 500 million and 1 billion doses in 2021 for global use.
The drugmaker has been working with the National Institutes of Health’s National Institute of Allergy and Infectious Diseases. The U.S. government committed to making nearly $1 billion available to Moderna for its vaccine research and other preparations and has agreed to pay about $3.1 billion to purchase 200 million doses.
As part of its Operation Warp Speed, the U.S. provided similar amounts to a range of vaccine developers, concluding that potentially wasting money was a reasonable trade-off to get vaccines available early to save lives.
The FDA has insisted that vaccine makers meet high hurdles in their studies, but it still is speeding up the normal process for approving medical products. Because of the pandemic, the FDA is employing a faster process for granting an emergency-use authorization instead of the more time-consuming process of full approvals.
Peter Marks, the FDA official overseeing vaccines, said in a Monday public discussion with Howard Bauchner, editor of the Journal of the American Medical Association, that the NIH is contemplating a new study to answer a still-unanswered question: Apart from preventing symptomatic disease, can vaccines also curtail the transmission of infections from asymptomatic vaccinated people to others?
Moderna’s Covid-19 Vaccine Could Widen Immunization Effort
The vaccine can be more easily shipped and handled than Pfizer’s, helping smaller hospitals and rural areas overcome logistical hurdles.
Health officials across the U.S. are counting on the arrival of a second Covid-19 vaccine to boost scarce supplies and sidestep logistical issues encountered by the first vaccine, which began distribution this week.
The U.S. Food and Drug Administration may issue an emergency authorization for a vaccine from Moderna Inc. as early as Friday after an advisory panel recommended the agency approve its use. If authorized, Moderna’s vaccine will join a vaccine from Pfizer Inc. and BioNTech that received authorization on Dec. 11.
The green light would nearly double this month’s expected U.S. supply of Covid-19 vaccine doses and help meet a federal goal of getting a vaccine to anyone who wants one by the spring or summer of 2021. Moderna expects to add 20 million doses of its vaccine to Pfizer’s expected U.S. supply of 25 million in December.
“The addition of the Moderna vaccine to the response will be huge,” said Claire Hannan, executive director of the Association of Immunization Managers, whose members direct state vaccination efforts.
Not only will the Moderna vaccine boost the supply of doses, but also it will “be much easier to send [it] to smaller providers and rural areas,” she said.
Moderna’s vaccine has easier storage and handling requirements and will be shipped in smaller quantities, health officials say. It can be stored in most standard medical freezers, while Pfizer’s must be shipped and kept at ultracold temperatures requiring either specialized freezers or dry ice, resources more common in large hospital systems and urban areas.
Once thawed, Moderna’s vaccine can be kept refrigerated for 30 days, while Pfizer’s can stay refrigerated for only five days after thawing.
Moderna’s vaccine also can be shipped in containers with as few as 100 doses, while Pfizer’s minimum order size is about 975 doses.
Pfizer’s larger minimum order size “poses challenges especially in rural areas of the country where that volume of product is more difficult to manage,” Anita Patel, deputy of the vaccine task force at the Centers for Disease Control and Prevention, said during an FDA advisory panel meeting on Dec. 10.
“This ultracold really, really makes it difficult to plan, based on the large quantity, and then also the ability of our partners to vaccinate during that time frame,” said Rich Lakin, immunization director for the Utah Department of Health. It is directing doses to hospitals, local health departments and pharmacies in the state.
Both vaccines are highly effective in preventing Covid-19 and use a similar gene-based technology. Pfizer’s vaccine was 95% effective at preventing disease in a study of 44,000 people, while Moderna’s was 94.1% effective in a 30,000-person study. Both vaccines were developed and tested at unprecedented speed.
Both vaccines also require people to come back three or four weeks later for a second dose. However, Moderna’s can be injected into people as is, while Pfizer’s has to be diluted in a separate solution before injection.
“Moderna definitely makes it easier from a logistics standpoint,” said Kristen Ehresmann, director of the infectious disease division with the Minnesota Department of Health. “It will help to fill in gaps for greater Minnesota, for the more rural parts of our state. Some areas of the state may not have access until the Moderna vaccine comes along.”
Federal and state officials said they have shaped their distribution strategies around the different features of the vaccines.
A Pfizer spokesman said the company’s supply-chain infrastructure is designed to ensure that people can get access to vaccine doses that meet its temperature requirements. A Moderna spokesman said the company has worked to improve the storage and shipping requirements for its vaccine, and stability testing this year has shown that it can be kept at higher temperatures.
A Covid-19 vaccine authorization would be Moderna’s first government clearance for a drug or vaccine in the 10 years since the company was formed to try to exploit an emerging gene-based technology. Anticipation of the Covid-19 vaccine has catapulted Moderna into the ranks of the most highly-valued drug companies, giving it a market capitalization of $54 billion.
The authorization would also validate the gene-based technology that Moderna has used to develop more than 20 other products for various diseases. FDA approval would signal that the technology could yield future vaccines and drugs using the same basic building blocks behind the Covid-19 vaccine.
Moderna and Pfizer are ramping up production, but so far the U.S. has committed to buying more of Moderna’s vaccine. On Dec. 11, the U.S. government agreed to double its purchase of Moderna’s vaccine to 200 million doses by the end of June. Pfizer has agreed to supply 100 million doses, though federal officials are in talks to secure additional doses, Secretary of Health and Human Services Alex Azar told reporters on Wednesday.
Initially, when supplies are limited, both vaccines are being reserved for health-care workers and residents of nursing homes and other long-term care facilities. As more doses become available, next in line are expected to be other essential workers, like police and teachers, senior citizens and people with underlying health conditions that put them at higher risk of severe Covid-19 disease.
Additional vaccines could also boost the mass immunization campaign if they prove successful in testing and are authorized for use.
Johnson & Johnson is testing a single-dose vaccine and expects to have results of a study of its vaccine in more than 40,000 people in January. That vaccine, unlike Pfizer’s and Moderna’s, wouldn’t require patients to take a second dose, and the company may seek U.S. authorization in February, if results are positive.
Updated: 12-08-2020
FDA Says Pfizer-BioNTech Vaccine Is Safe, Effective
Favorable analysis paves way for approval as early as this weekend.
The Food and Drug Administration said the first Covid-19 vaccine being considered for U.S. distribution “met the prescribed success criteria” in a clinical study, paving the way for the agency to green-light distribution as early as this weekend.
An outside panel of scientific advisers will review the FDA report Thursday, along with a companion analysis from the vaccine’s manufacturers, Pfizer Inc. and German partner BioNTech SE. BNTX 1.92% A favorable recommendation from the panel is expected to be followed within a few days by the FDA granting emergency authorization for the vaccine.
In its report Tuesday, the FDA noted that the two-dose vaccine provided benefits even after just the first injection—cutting the risk of getting Covid-19 by about half. The vaccine was found to be 95% effective after the second dose, three weeks later.
FDA scientists also found that the vaccine was effective in reducing the risk of confirmed severe disease after the first dose, an important finding as some health experts were concerned Covid-19 vaccines would protect against only mild to moderate disease.
Side effects were common, however, especially in younger people, the analysis found. The most common complaint was fatigue, followed by muscle pain and joint pain.
Severe “adverse reactions” were rare, most frequent after the second dose, and generally less frequent in older adults greater than 55 years of age.
The frequent side effects occurring soon after injections suggests that the vaccine is generating a strong immune response, said Dr. Angela Rasmussen, a virologist and affiliate at the Center for Global Health Science and Security at Georgetown University.
“It doesn’t mean the vaccine is making you sick, but you should be prepared for potentially not feeling great for a day or two after getting it,” she said.
Vaccines are seen as key to stopping the spread of the coronavirus, and health officials have stressed the importance that they be effective in high-risk populations such as minority groups and the elderly that have been hit hardest by the pandemic.
Dr. Anthony Fauci, the U.S. government’s top infectious-disease expert, said Tuesday that people’s hesitancy to get vaccinated could be a big hurdle.
“There are a substantial proportion of people who do think this is not real, that it’s fake news, or it’s a hoax. This is extraordinary. I’ve never seen this before,” Dr. Fauci said at The Wall Street Journal’s CEO Council Summit on Tuesday.
Dr. Anna Durbin, a vaccine researcher at Johns Hopkins University, who is helping test a Covid-19 vaccine being developed by AstraZeneca PLC, said it would be important to see more participation of Black people in future Covid-19 vaccine studies.
“There’s a lot of distrust in the [Black] community around research in general,” she said.
At the same time, Dr. Durbin said it was encouraging that the vaccine appeared to provide some protection 10 days after the first dose.
The information released Tuesday is likely to build confidence among health authorities and physicians preparing for a mass vaccination campaign. States, hospitals and other vaccination sites that will receive shots early on are readying themselves and building vaccination teams as Pfizer nears potential distribution.
Pfizer and BioNTech previously said the vaccine was shown to be 95% effective at protecting against symptomatic Covid-19.
Albert Bourla, Pfizer chief executive, said during an industry group panel on Tuesday that while the vaccine was developed quickly, it went through rigorous testing and followed strict standards, even more so than normal given the scrutiny during the pandemic.
“We didn’t cut any corners,” he said.
The analyses released Tuesday offer new insight into how effective the two-dose shot might be, as well as how safe it could be should regulators clear it for use in the U.S.
Tuesday’s data also gave new details on the two months of safety data on about 19,000 vaccinated study subjects requested by the FDA. The agency said trial participants who were given the vaccine were more likely to get certain side effects than patients who received the placebo. The most common were injection site reactions, fatigue, headache, muscle pain, chills, joint pain and fever.
Still, much remains unknown about the vaccine because it has been studied only for several months. Typically, vaccines take years to develop and test before getting a green light by governments.
The FDA found more testing will be needed to confirm that the vaccine effectively prevents death. And its analysis concluded that the data was limited in showing whether it can curb transmission of Covid-19 from individuals who are infected despite vaccination.
The agency said that the vaccine was found to be effective across race, age and ethnicity, and that its analyses found no safety concerns in subgroups by race, age, ethnicity or people with comorbidities such as pre-existing conditions.
On Thursday, however, the outside panel may scrutinize the demographic breakdown of the trial, as health authorities have sought for Covid-19 vaccine trials to include diverse populations. More than 80% of the approximately 38,000 trial subjects for whom Pfizer submitted data were white.
Pfizer’s late-stage trial began in July, enrolling some 44,000 people at more than 100 sites around the world.
The efficacy was determined after 170 subjects became sick, triggering a review by an outside panel of experts that found that most of those subjects had received a placebo rather than the vaccine. Researchers looked at how well the shots worked seven days after a study volunteer got a second dose.
The sick participants included 10 whose cases of Covid-19 were severe. Nine had received the placebo and one the vaccine.
Data disclosed Tuesday showed that the vaccine was effective across ages, weights and races, although most of the sick subjects were white and many were 55 years old and younger.
Of the sick subjects, 162 received a placebo and eight received the vaccine. The sick subjects who were white totaled 153, with 146 receiving a placebo and the rest getting a vaccine, resulting in about 95% efficacy within that subgroup.
The vaccine was found to be 100% effective in Black people, with seven Black subjects becoming sick, all of whom were in the placebo group.
Of the sick subjects aged between 16 and 55, 114 received the placebo and five received a vaccine.
Six subjects died during the trial, with two receiving the vaccine and four in the placebo group. None of the deaths were concluded to be related to the vaccine, according to Pfizer. The FDA said the deaths “represent events that occur in the general population of the age groups where they occurred, at a similar rate.”
The vaccine uses an innovative gene-based technology known as messenger RNA that has never been approved to prevent any infectious diseases.
Messenger RNA, or mRNA, teaches cells to make something resembling the spike protein that juts from the surface of the new coronavirus. Exposure to the protein preps a vaccinated person’s immune system to fight off the virus.
In its own briefing document, Pfizer said its program with BioNTech “has ensured the highest compliance and quality standards while progressing expeditiously to address this urgent and unmet medical need.”
The FDA has held advisory-committee hearings on hundreds of products over the years, but rarely has there been the kind of intense interest as in this week’s planned hearing on Thursday. This is largely due to the fast increase in cases of the illness, which has created the largest pandemic in a century.
Johns Hopkins on Monday reported that U.S. cases were approaching 15 million, and that U.S. deaths already topped 283,000.
The FDA has sped up its traditional monthslong approval processes by considering Pfizer’s Covid-19 vaccine for emergency-use authorization. These authorizations, in essence, involve balancing risks and benefits.
But because vaccines, unlike most drugs, are given to healthy people, the FDA has still insisted on rigorous standards—including convening Thursday’s public meeting and making public the companies’ and agency’s analyses.
With a 95% efficacy, Pfizer’s vaccine exceeded the FDA’s request that a vaccine had to lower the rate of disease by 50% or more when compared with a placebo. The FDA also said that half of patients in any vaccine study would need to be followed for at least two months after inoculation to ensure that major side effects didn’t occur and that the vaccine’s effectiveness lasted.
If authorized, Pfizer would begin shipping the first of 25 million doses this year, the equivalent for 12.5 million people because it requires two doses. Vaccinations in the U.K. began Tuesday.
Federal officials have estimated that U.S. vaccine deliveries during December will be enough for about 20 million people. That compares with 24 million people in the U.S., such as health-care workers and residents of long-term care facilities, who are in priority groups for vaccines.
Updated: 11-23-2020
Astra-Oxford Vaccine Found Effective In Preventing Covid
A Covid-19 vaccine developed by the University of Oxford and AstraZeneca Plc prevented a majority of people from getting the disease in a large trial, another promising development in the quest to end the pandemic, and the rollout could begin next month.
The vaccine stopped an average of 70% of participants from falling ill, an early analysis of the data show. The effectiveness rose to 90% for one of two regimens, using half a dose followed by a full one later, close to the high bar set by Pfizer Inc. and Moderna Inc.
Astra and Oxford officials said they’re preparing to submit the findings to regulators and don’t expect the different outcomes in the study to affect the process. The U.S. could potentially take longer to sign off because a clinical trial in that country will need more time before it delivers results.
— University of Oxford (@UniofOxford) November 23, 2020
“Our goal was to make sure we can have a vaccine that is accessible everywhere,” Andrew Pollard, who is leading the Astra-Oxford trials, said Monday at a press briefing. “I think we have actually managed to do that.”
The results, based on trials in the U.K. and Brazil, were reviewed after 131 participants contracted Covid-19. The full two doses showed an efficacy of 62%. Among those who received the vaccine, there were no severe cases and no participants were hospitalized. The group is planning to submit the data for peer review in the next 24 hours.
Astra shares dropped as much as 4.2% in London, losing ground after some analysts questioned the data, including the fact that the subset of the trial showing 90% efficacy included fewer patients. The stock is still up about 30% since mid-March, but Moderna and Pfizer shares surged when they reported their results.
Ruud Dobber, head of Astra’s biopharmaceuticals business, said in an interview with Bloomberg Television that it’s too early to speculate about how regulators will react.
Moderna shares rose 1.7% and Pfizer was down 1.1% at 11 a.m. in New York.
The larger U.S. Astra trial, which could be key for approval there, has injected about 10,500 people with both doses. Mene Pangalos, Astra’s head of biopharma research, told reporters the team is planning to talk to the U.S. Food and Drug Administration immediately and hopes to have another arm administering the half dose-full dose regimen starting within weeks.
“That scenario is possible,” that other regulators could move before the U.S., he said. “We need to share the data with the FDA, which we will do very quickly, and then we will work out what the most appropriate steps are.”
Despite the apparently lower efficacy than shots from Pfizer and Moderna, which each prevented about 95% of cases, the British vaccine has some advantages. Their shot can be kept at refrigerator temperatures, while those from Pfizer and Moderna, based on novel messenger RNA technology, require freezing for longer-term storage and transport. That would make Astra’s easier to deploy globally, particularly in lower- and middle-income countries. It also comes at a lower cost.
The Astra-Oxford team cautioned against comparing the efficacy levels of the vaccines too closely at this stage. Pam Cheng, who runs Astra’s global operations and is overseeing the manufacturing of the vaccine, said the half dose-full dose regimen shouldn’t affect global production or supplies of the shot, other than to potentially increase the number of doses available.
AstraZeneca expects to have more than 300 million doses ready to ship globally by the end of the first quarter of next year, with about 100 to 200 million doses being produced monthly. For the U.K., the company expects to have up to 4 million doses ready by year-end, and 40 million by the end of the first quarter.
The differing results of the two regimens may leave questions about the best way to give the AstraZeneca shot. Analysts at Barclays Plc had put consensus expectations for what would be deemed a success from AstraZeneca at 70% to 90%, following conversations with investors in Europe and the U.S.
AstraZeneca said it will immediately prepare to submit data to authorities around the world that have a framework in place for early approval. The company said it will seek an emergency use listing from the World Health Organization for an accelerated pathway to vaccine availability in low-income countries.
Updated: 11-16-2020
Moderna’s Covid-19 Vaccine Is 94.5% Effective In Early Results, Firm Says
Awaiting fuller safety data later this month, Moderna said it plans to seek federal authorization for shot by early December.
Moderna Inc. MRNA 9.58% said its experimental coronavirus vaccine was 94.5% effective at protecting people from Covid-19 in an early look at pivotal study results, the second vaccine to hit a key milestone in U.S. testing.
Ninety-five people in the study developed Covid-19 with symptoms; of those, 90 had received a placebo and only five Moderna’s vaccine.
The findings, from a 30,000-subject trial that is still under way, move the vaccine closer to wide use, because they indicate it is effective at preventing disease that causes symptoms, including severe cases.
The vaccine also showed signs of being safe, though researchers and regulators must wait for more-complete safety data from the study, expected later in November. Moderna said it plans to ask federal health authorities by early December to clear the vaccine.
How Messenger RNA Vaccines Work
The experimental vaccine from Moderna, as well as the candidate from Pfizer and BioNTech, use a new gene-based technology known as mRNA.
If greenlighted, the shot could go into distribution that month, making it one of the first Covid-19 vaccines to go into distribution in the U.S., where reported coronavirus cases and hospitalizations are surging.
“We may be in a place with a vaccine that has a big impact on the prevention of severe disease,” Moderna Chief Executive Stéphane Bancel said in an interview. “That will be an incredible win against this awful virus.”
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said the high efficacy rate for Moderna’s vaccine means it could be an effective tool to help end the pandemic.
“These are very impressive and very encouraging and exciting results,” Dr. Fauci said.
Earlier this month Pfizer Inc. and BioNTech SE said their experimental Covid-19 vaccine was more than 90% effective at protecting people from symptomatic Covid-19 in a large clinical trial. Pfizer plans to ask the U.S. Food and Drug Administration this month to authorize use of the shot, setting up potential distribution to begin by the end of the year.
Though preliminary, the early results for the two vaccines suggest researchers can develop effective Covid-19 shots, which would be a big help in taming the pandemic.
“I think the high efficacy and data on severe cases mean that these RNA vaccines should markedly reduce the burden of hospitalization from Covid-19,” said Larry Corey, a vaccine specialist at the Fred Hutchinson Cancer Research Center in Seattle, and head of a network of clinical trial researchers who helped test the Moderna vaccine.
Moderna’s initial data were reviewed by a committee of independent researchers monitoring the study. The findings provide only a rough snapshot of whether the vaccine works. The company plans to release additional results later, including effectiveness in specific groups such as the elderly and against infections that don’t produce symptoms.
Researchers won’t have a full set of effectiveness data until a total of 151 people in the trial develop Covid-19 symptoms. Moderna expects that by the time it applies for government authorization, Mr. Bancel said.
The company said the efficacy rate could change when more cases are analyzed, but the early numbers surpassed the high bar it had set for preliminary results.
Both the Moderna and Pfizer Covid-19 vaccines are among the most advanced in development in the West, along with candidates from AstraZeneca PLC, Johnson & Johnson and Novavax Inc.
Covid-19 vaccines developed in China and Russia have already been administered, though they haven’t finished the final stage of testing. Russia said last week its vaccine had 92% efficacy in a large trial.
Moderna’s vaccine, like Pfizer’s, uses a new technology known as messenger RNA. It works by delivering genetic instructions that teach human cells to make a protein resembling one found on the surface of the coronavirus. That triggers an immune response designed to protect vaccinated people if they are later exposed to the actual virus.
Moderna, a Cambridge, Mass., biotech that has been an mRNA pioneer, has never brought a drug or vaccine to market and no mRNA vaccine has ever been cleared by regulators. The National Institute of Allergy and Infectious Diseases worked with the company to develop its vaccine.
The federal government, under the Operation Warp Speed initiative, has committed about $2.5 billion to Moderna to support its vaccine research and testing and to buy at least 100 million doses of the vaccine.
In the Phase 3—that is, late-stage—study of its vaccine, people at about 100 U.S. locations were given two doses of the vaccine or a placebo, four weeks apart. Researchers tracked cases of symptomatic Covid-19 starting at least two weeks after the second dose.
The study’s design called for the independent committee to conduct the first interim analysis of efficacy when 53 people came down with symptomatic Covid-19. Moderna said the first analysis involved substantially more people because of the recent increase in Covid-19 cases nationally.
Among the 95 cases reviewed, 11 were severe, all in people receiving the placebo, Moderna said.
Moderna said the 95 Covid-19 cases included 15 people ages 65 years and older, and 20 people from diverse communities such as Hispanic or Latinx and Black people.
Because of the urgent need, the FDA plans to clear Covid-19 vaccines more quickly than is standard in a public health emergency. Technically, the authorization would be for emergency use, rather than a standard approval.
The FDA wants to see two months of safety monitoring for at least half of the 30,000 people in the trial, a milestone Moderna expects by the end of November. In the early look, the company said no significant safety concerns were reported, and the vaccine was generally well tolerated, with injection-site pain for some people after the first dose, and fatigue, headache and joint pain after the second.
If regulators do authorize the vaccine, the initial supply of doses will be limited—20 million, or enough for 10 million people, by the end of the year, Moderna forecasts. If the Pfizer vaccine is also authorized, federal officials said Monday they expect to be able to immunize about 20 million Americans during December.
The federal government may decide to vaccinate health-care workers and first responders first, followed by other segments of the population in phases until more doses are made next year.
Plans haven’t been finalized, but a National Academy of Medicine committee has recommended that the first phase of vaccination cover about 5% of the population and include front-line health workers such as those in hospitals and nursing homes, workers who provide transportation and other services to health-care facilities, and first responders.
The next phase would cover 10% of the population and include people with health conditions that put them at higher risk of severe Covid-19 disease or death, and people age 65 and older who live in settings such as nursing homes, long-term care facilities, homeless shelters and prisons.
Moderna is working with contract manufacturers to boost production so it can make 500 million to one billion doses next year. Its U.S. contract has an option for 400 million doses in addition to the initial 100 million. Moderna also plans to seek authorization in other countries, and has signed supply contracts with several.
Federal health officials cautioned Monday that despite the positive data for the vaccines, the limited initial supply also means that Americans should continue to take precautions against the coronavirus, such as wearing masks and washing hands.
Updated: 11-12-2020
Moderna Poised To Take Vaccine Spotlight With Data Due
The same U.S. explosion of Covid-19 cases that helped Pfizer Inc. get results for its vaccine trial earlier this week is helping speed along Moderna’s trial. Moderna said Wednesday its study has accumulated more than 53 infections, allowing a preliminary analysis of the shot’s effectiveness to begin. The shares jumped.
Moderna didn’t predict how long it could take an independent monitoring committee to analyze the data, but said the company could get the data to the committee within days. The company said it is still blinded to the data.
“Moderna has seen a significant increase in the rate of case identification across sites in the last week,” the company said in a statement. “As a result, the company expects the first interim analysis will include substantially more than 53 cases, the targeted trigger point for the analysis.”
The preliminary data on Moderna’s study is being prepared for submission to the monitoring board, Moderna said. The board will say whether the vaccine is effective, doesn’t work, or that the trial should continue because the results are inconclusive.
The bet among top experts in the field is that Moderna’s therapy, which uses a similar mRNA technology to Pfizer’s, will likely prove to be highly effective, perhaps mirroring Pfizer’s announcement earlier this week that its shot appears to be more than 90% effective.
“Overall I would expect similar results” in Moderna’s trial, said Drew Weissman, an immunologist and mRNA expert at the University of Pennsylvania who helped develop key modifications used in mRNA vaccines.
“It is hard to imagine how it would be much different,” according to Weissman, whose lab receives research funding from BioNTech SE, the company partnering with Pfizer on its vaccine.
Certain Numbers
In vaccine trials, a certain number of volunteers — a percentage of which get a placebo — have to get infected in order to determine if the vaccine works. That’s easier to accomplish with the pandemic in the U.S. hitting record infections on a daily basis.
Pfizer got a burst of results in recent weeks that pushed that trial over the line to take a first look. Now Moderna’s interim analysis could come within days.
Whatever happens in the trial — and there are no guarantees until it is done — the results are certain to have a big impact on Moderna shares, which have more than quadrupled this year in a wild roller-coaster ride. The stock closed up 8.4% in New York trading at $82.44 Wednesday. On Thursday, the shares rose another 5% in pre-market trading.
A preliminary analysis in Moderna’s trial would likely have already been available if the shot was only 60% effective, according to the research firm Airfinity Ltd. If the Moderna vaccine turns out to be 90% effective, however, that time line would be expanded and Moderna would be getting results around now, Airfinity said.
The more effective a vaccine is, the longer it takes for cases to add up since there would be fewer infections in the half of participants who got the vaccine rather than a placebo.
The strong similarity with the successful Pfizer vaccine is boosting confidence in Moderna’s, which was developed in concert with the U.S. National Institute of Allergy and Infectious Diseases.
The Pfizer result “validates the mRNA platform,” said Anthony Fauci, director of the NIAID and the U.S. government’s top infectious disease doctor, in a call with reporters Monday. “Moderna is an mRNA candidate, which we would expect to have similar results.”
Dose Differences
Moderna’s final-stage trial started on the same day as Pfizer’s big trial in late July. The company is slightly behind Pfizer largely due to structural differences in the trials. The two doses of Moderna’s vaccine are given four weeks apart, instead of the three-week gap used in for Pfizer’s vaccine.
In addition, Moderna doesn’t start counting coronavirus cases until 14 days after the second vaccine dose, as opposed to 7 days after the second dose in the Pfizer trial. Pfizer’s trial also has more participants.
Pfizer originally planned to analyze its results after a mere 32 cases had occurred, but the plan to peek at the results so early on was controversial among medical experts. The drugmaker agreed with the U.S. Food and Drug Administration to wait until 62 cases occurred in its trial before starting to analyze the results. By the time it began crunching the data, though, a total of 94 cases had come in.
CureVac Aims To Overcome Deep-Freeze Challenge With Covid Shot
CureVac NV, the German biotech company, said its experimental coronavirus vaccine may be able to overcome storage and distribution challenges that threaten to hinder the rollout of rival shots.
Data show CureVac’s messenger RNA vaccine remained stable for at least three months when stored at a standard refrigerator temperature of 5 degrees Celsius (41 degrees Fahrenheit), and for up to 24 hours as a ready-to-use shot when stored at room temperature, the company said Thursday.
Some vaccines, like the front-runner from Pfizer Inc. and BioNTech SE, must be kept at ultra-cold temperatures and require complex handling to remain viable. That storage requirement is an obstacle that will need to be tackled before the vaccine can be made broadly available, according to experts, and the deployment costs raise worries that poorer nations will be left behind.
CureVac said its stability study is ongoing, aimed at further evaluating the potential for a longer commercial shelf-life.
Speaking at an event Thursday, Anthony Fauci, the top U.S. infectious disease official, pointed to the next vaccines in the pipeline that won’t have the need to be stored at such cold temperatures.
Updated: 11-9-2020
Pfizer’s Covid-19 Vaccine Proves 90% Effective In Latest Trials
Drugmaker and partner BioNTech could seek FDA authorization by end of November.
A vaccine developed by Pfizer Inc. and partner BioNTech proved better than expected at protecting people from Covid-19 in a pivotal study, a milestone in the hunt for shots that can stop the global pandemic.
The vaccine proved to be more than 90% effective in the first 94 subjects who were infected by the new coronavirus and developed at least one symptom, the companies said Monday.
The positive, though incomplete, results bring the vaccine a big step closer to getting cleared for widespread use.
Pfizer said it is on track to ask health regulators for permission to sell the shot before the end of this month, if pending data indicate the vaccine is safe.
The timetable suggests the vaccine could go into distribution this month or next, though U.S. health regulators have indicated they will take some time to conduct their review. Then it will take months for the companies to make enough doses for the general population.
“Hopefully now we can move on and get this vaccine out there and make sure it’s doing what it’s supposed to do and stop” the virus, said Kathrin Jansen, Pfizer’s head of vaccine research and development, in an interview.
The findings arrived on the timetable that the companies had been projecting. The results came too early for researchers to assess the safety of the vaccine, which the U.S. Food and Drug Administration says must include two months of monitoring at least half the study’s subjects for side effects.
Pfizer said it remained on track to collect at least two months of safety data during the third week of November and could file for an emergency authorization shortly thereafter.
So far, no serious safety issues have arisen in the study, the companies said. The study has enrolled nearly 44,000 subjects in the U.S. and other countries.
It is unclear how long the protection the vaccine appears to provide lasts, since researchers haven’t been studying volunteers for very long.
“You have to be encouraged by this,” said Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, who is on an FDA vaccine-advisory committee. “It certainly looks like things are trending in the direction for protection.”
Dr. Offit said it would be important to learn how well the vaccine works in high-risk groups, such as the elderly and diverse populations. Researchers say that in order for vaccines to be effective they must work across age groups, races and ethnicities.
The interim review of the vaccine’s effectiveness and safety was performed by an outside panel of independent experts known as a data-safety monitoring committee, which then shared its findings with Pfizer and BioNTech.
“You never know what the outcome is, but we had a feeling that we did everything we could possibly do,” Dr. Jansen said.
The vaccine is among the most-advanced in development in the West, with others in late-stage testing from Moderna Inc., Johnson & Johnson and AstraZeneca PLC.
Covid-19 vaccines developed by researchers in China and Russia have already been given to people in those and certain other countries.
The positive results don’t mean other vaccines will also prove to work, but they do suggest people can be protected against Covid-19.
“The most important message is that you can make a vaccine against this critter,” said Prof. John Bell, a health-policy adviser to the U.K. government from the University of Oxford who led Oxford in reaching its Covid-19 vaccine deal with British drugmaker AstraZeneca PLC.
Prof. Bell predicted that Oxford and AstraZeneca “won’t be far behind” in releasing their late-stage trial results out of the U.K. The Oxford-AstraZeneca results are expected within weeks.
Pfizer and BioNTech’s vaccine uses a new and unproven technology, known as mRNA, short for the molecular couriers called messenger RNA that carry genetic instructions to cells.
The shots deliver mRNA that prompts cells to make a synthetic version of the spike protein that juts from the surface of the new coronavirus. That protein triggers the immune system to defend against the virus.
Moderna’s vaccine also uses the mRNA technology. “This is great news as it shows mRNA can work,” Moderna Chief Executive Stéphane Bancel said. “Great day for the world, as we are all waiting for vaccines.”
He said that given the high Covid-19 cases rate in the U.S., Moderna is on track for an interim efficacy analysis and a two-month safety follow-up for at least half the people in its clinical trial this month.
After its vaccine appeared to work safely in a smaller and earlier-stage study, Pfizer and Germany’s BioNTech began in July seeking thousands of healthy volunteers for the large final-phase trial to determine whether it could be given to the public.
Like most vaccine trials, just a fraction of the subjects must become sick to evaluate whether the two-dose shot from Pfizer and BioNTech works.
For the final analysis, 164 study subjects need to become infected and develop at least one symptom. Researchers, however, designed the trial to take peeks at how the shot is performing after smaller numbers get sick.
Researchers originally planned for a first interim analysis after 32 subjects became sick. After talking with the FDA, Pfizer agreed to conduct the early peek after at least 62 subjects became sick, Dr. Jansen said.
By the time the two sides came to an agreement, the number of subjects who developed Covid-19 symptoms reached 94, Dr. Jansen said.
Pfizer officials learned about the early, or interim, analysis Sunday after speaking with the data-safety monitoring committee, Dr. Jansen said.
She said Pfizer has shared the outcome of the analysis with the FDA, though the World Health Organization and the European Medicines Agency, the region’s health regulator, said they haven’t seen the data yet.
The EMA has said it would clear a vaccine for use in the European Union even if it isn’t 50% effective and the WHO had set similar expectations, while the FDA has said it won’t authorize a vaccine unless it is at least 50% effective. The U.S. agency and companies wanted to see an even higher rate during an early look at an initial set of subjects to be sure it really works.
In its first look, however, the Pfizer and BioNTech vaccine worked even better than the FDA and two companies had been seeking.
The two-dose vaccine was found to be more than 90% effective at seven days after the second dose, Pfizer said, meaning that subjects were protected four weeks after their first shot.
Pfizer didn’t disclose the breakdown of how many of the 94 subjects in the analysis received the vaccine or a placebo. In the study, half receive the vaccine, while the other half receive a placebo.
While the case split of vaccine and placebo breakdown wasn’t available, the more than 90% efficacy rate suggests that most, if not all, of the 94 sick patients had received the placebo.
Although specific safety information wasn’t available, Dr. Jansen said the data-safety monitoring committee told Pfizer officials that any side effects were similar to those in earlier testing of the vaccine.
Previously, Pfizer said some subjects in its early-stage study of the vaccine reported side effects such as fatigue, headaches and chills, and they eventually recovered. There weren’t serious side effects.
The latest timetable for the vaccine to become widely available is consistent with what Pfizer Chief Executive Albert Bourla and BioNTech co-founder and Chief Executive Ugur Sahin have suggested.
Pfizer plans to monitor patients for two years after their second dose for safety and vaccine duration.
Pfizer Covid-19 Vaccine: When Will It Be Ready and Everything Else You Need to Know
Initial effectiveness data was positive, yet more needs to be learned about the shot, including its safety, and manufacturing doses will take time.
Pfizer Inc. and BioNTech released promising, but preliminary, results for the effectiveness of their Covid-19 vaccine. Here’s what we know, and what we don’t know, and what this means for people getting the shots.
When Will The Pfizer Vaccine Be Ready For Authorization?
It will be several more weeks at the earliest, because researchers and regulators still need to make sure the shot is safe. The U.S. Food and Drug Administration has said it wants to see two months’ worth of safety outcomes after vaccination for at least half of the people participating in any large, final-stage clinical trial before it considers authorizing a Covid-19 vaccine.
The FDA says this will allow identification of any side effects, such as neurological or heart conditions, that weren’t apparent immediately after vaccination. So far, no serious safety issues have been found, Pfizer says. It expects the two months of safety data later this month, and can ask the FDA to authorize the vaccine soon thereafter. It isn’t yet clear how long the FDA will take to make a decision.
When Will People Start Getting Vaccinated?
Shots from Pfizer and BioNTech could start becoming available before the end of the year, as production has already begun, but initial supplies will be limited. Pfizer says it expects to produce up to 50 million doses globally in 2020—enough for 25 million people because the vaccine is given in two doses—and up to 1.3 billion doses in 2021.
This means only the highest risk groups, such as front-line health care workers, could be inoculated this year. Many more doses would be needed to cover the U.S. and global population. Other Covid-19 vaccines in development will likely be needed for everyone to get vaccinated. Their makers have projected they could produce billions of doses next year if their vaccines are successful in clinical testing.
What Does This Mean For The Timing Of Reopenings?
Pfizer and BioNTech had projected getting initial data around this time. Companies making other leading vaccine candidates also have forecast getting early data before year’s end. Given the limited initial supplies, however, public-health authorities have cautioned there wouldn’t be enough shots to vaccinate the general population until next year some time, perhaps the summer.
That means, the health authorities said, precautions such as social-distancing and mask-wearing will remain important for the foreseeable future, especially as cold weather drives many people indoors, and a return to normal won’t be for a while. “We actually need to redouble our efforts in the basics of infection prevention while we await therapeutics and vaccines,” said Dr. Lisa Maragakis, an infectious-disease specialist at Johns Hopkins University School of Medicine.
What Does This Mean For Other Covid-19 Vaccines In Development?
Pfizer’s analysis outcome bodes well for a Covid-19 vaccine developed by Moderna Inc., because it uses similar mRNA technology. Moderna expects efficacy and safety results from a large trial of its vaccine later this month. Other leading vaccines developed by AstraZeneca PLC, Johnson & Johnson and Novavax Inc. don’t use mRNA-based technologies, but they target the spike protein of the coronavirus that is also targeted by the mRNA vaccines.
These companies have started large Phase 3 trials and could have results within the next few months. “It seems to be a proof of principle that we can make a vaccine, that it can make an immune response, that it does look safe in individuals that have been studied so far and that it actually works in the real world to protect against Covid,” said Dr. Buddy Creech, director of the Vanderbilt Vaccine Research Program in Nashville, Tenn., said in an interview. “That’s all you can hope for in any vaccine. And for it to have occurred this quickly and this rigorously is really encouraging.”
What Don’t We Know Yet?
The effectiveness data announced by Pfizer and BioNTech was positive but preliminary. It left many unknowns beyond whether the vaccine is safe to take.
The analysis took place too soon for researchers to figure how long the protection conferred by the vaccine will last; the data only looked how well the vaccine worked seven days after subjects got a second shot. “What will be the protective efficacy over time?” Gregory Poland, director of the Mayo Clinic’s vaccine-research group. “Is this going to be a handful of months, like the flu vaccine?
Is it going to be like measles or smallpox where it’s lifelong immunity?” It also is unclear whether the vaccine worked at protecting against severe Covid-19 disease. Nor is it clear whether the vaccine worked in all the different populations that would be in line to get one, including the elderly, children and various racial and ethnic groups. Pfizer and BioNTech are expected to release additional data later.
Pfizer Vaccine’s Funding Came From Berlin, Not Washington
It’s said that success has many authors, and the encouraging data from Pfizer Inc.’s experimental Covid-19 vaccine had plenty of people in Washington lining up to take credit.
Vice President Mike Pence was among Trump administration officials saying support from the government’s Operation Warp Speed program helped accelerate the development of the vaccine, which was found to be more than 90% effective in preventing symptomatic Covid-19 infections in an interim analysis.
The truth is that Pfizer didn’t receive any funding from Operation Warp Speed for the development, clinical trial and manufacturing of the vaccine. Rather, its partner, BioNTech SE, has received money — from the German government.
BioNTech is credited for contributing the messenger RNA technology, which prompts the body to make a key protein from the virus, creating an immune response. The biotechnology company already had a history of working with Pfizer on influenza vaccines, and in March they clinched a deal to co-develop a shot to prevent against Covid-19 at research sites both in the U.S. and Germany.
The two companies began human testing of the vaccine in April, before the existence of Operation Warp Speed was revealed publicly.
Berlin gave the German company $445 million in an agreement in September to help accelerate the vaccine by building out manufacturing and development capacity in its home market.
What the U.S. did, meanwhile, was commit to buying hundreds of millions of vaccines in advance to ensure Americans were among the first in line if it clinches an emergency-use authorization or approval from the FDA. The Trump administration agreed in July to pay almost $2 billion for 100 million doses, with an option to acquire as many as 500 million more, once that clearance comes.
As part of that agreement, the U.S. gets to decide who gets the vaccine first, and will work with the company on logistical support. While most vaccine front-runners that have been tapped by Warp Speed will distribute their doses through a government partnership with McKesson Corp., Pfizer is handling its own delivery of its products.
The company has designed reusable containers that can keep the doses at ultracold temperatures, and is organizing trucks and flights to move them.
Operation Warp Speed is credited with speeding along several other vaccine programs, including one from Moderna Inc.that uses similar technology to Pfizer’s and could produce trial data later this month.
The Trump administration’s rapid-vaccine operation, led by the Health and Human Services Department, the Defense Department, and other agencies, could well prove to be the reason many Americans get a vaccine in 2021, even if it’s not made by Pfizer.
Some Republicans, including Donald Trump Jr. and Texas Senator Ted Cruz, questioned the timing of Pfizer’s release of its positive data on Monday, almost a week after the presidential election — with the implication that the information could’ve changed the outcome and tipped the scales toward President Donald Trump, who lost to former Vice President Joe Biden.
Pfizer said on Oct. 27, a week before Election Day, that it hadn’t met the threshold for positive cases that would’ve allowed it to report the data. After that, it revised its trial protocols to raise that threshold higher, after consulting with the U.S. Food and Drug Administration on what would be acceptable to gain approval.
The FDA has been under pressure from scientists to set tough standards for a vaccine so that Americans will feel it has been rigorously vetted and is safe to use.
If Pfizer hadn’t raised its threshold in response to the FDA’s recommendations, it’s possible it could’ve hit the lower bar of 32 positive cases before the Nov. 3 election. But it’s unclear when the trial hit that number. The company didn’t find out it had surpassed the new, revised threshold of 62 positive cases until Sunday.
All along, Pfizer’s top executives have attempted to quell notions that it has been influenced by political players.
Chief Executive Officer Albert Bourla has repeatedly said that the drug giant has avoided taking taxpayer dollars for research and development purposes.
“I wanted to liberate our scientists from any bureaucracy,” Bourla said in an interview on CBS’s “Face the Nation” on Sept. 16. “When you get money from someone, that always comes with strings. They want to see how we are growing to progress, what types of moves you are going to do. They want reports. I didn’t want to have any of that.”
“Basically I gave them an open checkbook so that they can worry only about scientific challenges, not anything else. And also, I wanted to keep Pfizer out of politics, by the way,” Bourla added.
Updated: 10-25-2020
Gilead’s Remdesivir Becomes First Virus Treatment To Win FDA Nod
The U.S. Food and Drug Administration approved Gilead Sciences Inc.’s antiviral therapy remdesivir on Thursday, making it the first drug to obtain formal clearance for treating the coronavirus.
Regulators had granted an emergency-use authorization for remdesivir earlier this year, and since then the drug has become a widely used therapy in hospitalized Covid-19 patients. It was given to President Donald Trump this month when he was diagnosed with the virus.
The approval of remdesivir, sold under the brand name Veklury, will allow Gilead to market the drug and talk about its benefits to doctors, nurses, and patients. That could help solidify its position as a go-to medicine for Covid-19 patients even as other drugs for the disease begin to reach the market.
“Veklury is now the first and only approved Covid-19 treatment in the United States,” Gilead said in a statement. While the drug was in short supply initially, Gilead said that the medicine is now widely available in hospitals across the country as manufacturing capacity has rapidly expanded.
The drug hasn’t been proven to reduce deaths from Covid-19. In a World Health Organization trial, the medicine failed to reduce fatalities, according to preliminary results that were posted on preprint servers last week.
Gilead has criticized the WHO study. In a letter posted on the company’s website, Chief Medical Officer Merdad Parsey said the findings don’t negate other results.
Shares of Gilead gained 6.3% in pre-market trading on Friday. Analysts estimate that remdesivir will have sales of $2.17 billion this year, according to 13 surveyed by Bloomberg.
The company said in June that it will charge U.S. hospitals roughly $3,120 for most patients who need remdesivir.
The approval is based on a U.S. government-sponsored trial involving more than 1,000 hospitalized coronavirus patients that found that those who received the drug recovered about five days faster than those who got a placebo.
The overall side-effect rate was similar to the placebo in the government study. The most common side effects are nausea and elevated liver enzymes, according to the product’s label.
—————————
The Beijing branch of China’s National Health Commission said that a combination of lopinavir and ritonavir, sold under the brand name Kaletra by AbbVie, is part of its latest treatment plan for patients infected by the virus, which has killed at least 56 people in China and sickened more than 2,000 worldwide.


https://www.youtube.com/watch?v=r3miPW-wkfk&list=PLU1KxUVqGmaNg_rsd1ierCzedgtZpR1Ud

Coronavirus And MERS-CoV: Major Drugs In The Pipeline
The mysterious coronavirus outbreak in the Chinese city Wuhan and its fast spread across Asia, endangers thousands of lives. The pandemic has catalysed the development of novel vaccines across the biotech industry, both by pharmaceutical companies and research organisations such as the National Institutes of Health (NIH), US.
Here are five drugs that pharmaceutical companies across the world are developing that may have the potential to become major coronavirus vaccines or antivirals for treating the contagious coronavirus infection.
Novavax’s MERS Coronavirus Vaccine Candidate
Novavax developed a novel Middle East Respiratory Syndrome (MERS) coronavirus vaccine candidate in 2013, post the identification of the first MERS coronavirus ((MERS-CoV) in Saudi Arabia in 2012. It is a crucial target for vaccine development by the Coalition for Epidemic Preparedness Innovations (CEPI) and is a priority disease for the World Health Organisation (WHO).
The candidate is designed to primarily bind to the major surface spike (S) protein and developed using the company’s recombinant nanoparticle vaccine technology. Tested along with the Novavax’s proprietary adjuvant Matrix-M™, it inhibited infection by inducing immune responses in the laboratory studies.
The MERS coronavirus is related to the severe acute respiratory syndrome (SARS) coronavirus for which the company had previously developed a recombinant nanoparticle vaccine candidate.
Inovio Pharma’s INO-4700, A Potential Coronavirus Vaccine
The investigational DNA immunotherapy, INO-4700 (GLS-5300) is being developed by Inovio in partnership with GeneOne Life Science. It is delivered as vaccine intramuscularly, using the Cellectra® delivery device.
The vaccine was well-tolerated and demonstrated high immune responses against the MERS-CoV in 94% of patients in the early-stage clinical trial in July 2019.
It also generated broad-based T cell responses in 88% of the subjects.
“Research organisations such as the National Institutes of Health (NIH), US are also developing a vaccine for the coronavirus.”
Biocryst Pharma’s Galidesivir, A Potential Antiviral For Coronavirus Treatment
The antiviral drug Galidesivir (BCX4430) has shown broad-spectrum activity against a wide range of pathogens including coronavirus. It is a nucleoside RNA polymerase inhibitor that disrupts the process of viral replication.
The drug has already shown survival benefits in patients against deadly viruses such as Ebola, Zika, Marburg, and Yellow fever.
Galidesivir is currently in advanced development stage under the Animal Rule to combat multiple potential viral threats including coronaviruses, flaviviruses filoviruses, paramyxoviruses, togaviruses, bunyaviruses, and arenaviruses.
AbbVie’s Lopinavir For MERS-CoV And SARS Coronavirus
An HIV protease inhibitor, lopinavir is being studied along with ritonavir for the treatment of MERS and SARS coronaviruses. The repurposed drug is already approved for the treatment of HIV infection under the trade name Kaletra®.
The combination is listed in the WHO list of essential medicines. Lopinavir is believed to act on the intracellular processes of coronavirus replication and demonstrated reduced mortality in the non-human primates (NHP) model of the MERS.
Lopinavir/ritonavir in combination with ribavirin showed reduced fatality rate and milder disease course during an open clinical trial in patients in the 2003 SARS outbreak.
Regeneron’s REGN3048-3051 For Coronavirus Infection
Discovered by Regeneron, the combination of neutralising monoclonal antibodies REGN3048 and REGN3051 is being studied against coronavirus infection in a first-in-human clinical trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). The safety and tolerability of the drug will be studied in 48 patients.
Both the antibodies bind to S-protein of MERS coronavirus. The intravenous administration of the drug in the mouse model of MERS resulted in the high-level neutralisation of the MERS coronavirus in circulating blood with reduced viral loads in the lungs.
Human Coronavirus (229E) / SARS-CoV / MERS-Cov
The contagious coronavirus outbreak at the end of 2019, which the WHO named as 2019-nCoV, led to a medical emergency in China.
Caused by human alpha and beta coronaviruses such as 229E, NL63, OC43 and HKU1, the infection is responsible for SARS and MERS.
Coronavirus transmission
“Coronavirus transmission can happen human-to-human as well as from infected animals such as dogs and cats.“
The viruses, believed to have transmitted from animals and reptiles such as snakes, cause respiratory issues such as upper respiratory tract illnesses and lower respiratory illnesses such as pneumonia and bronchitis.
Coronavirus transmission can happen human-to-human as well as from infected animals such as dogs and cats.
Updated: 1-27-2020
Montco Biopharm Firm Gets $9M Grant To Battle China’s New Coronavirus
The Coalition for Epidemic Preparedness Innovations (CEPI) has awarded a $9 million grant to Inovio Pharmaceuticals to support development of a vaccine against the recently emerged strain of coronavirus.
The new coronavirus strain has killed 17 people, and infected nearly 600 more, in China.
The CEPI funding will support Inovio’s preclinical and clinical development, through phase-I human testing, of INO-4800 – the Plymouth Meeting company’s new potential coronavirus vaccine matched to the outbreak strain.
The vaccine candidate is based in part on technology generated in the lab of David B. Weiner, executive vice president and director of the Vaccine & Immunotherapy Center at The Wistar Institute. Wistar researchers are working with Inovio in its efforts to develop a vaccine for the new coronavirus strain.
CEPI, a public-private alliance formed in 2016 to finance and coordinate the development of new vaccines to prevent and contain infectious disease epidemics, previously awarded Inovio (NASDAQ: INO) a grant of up to $56 million for the development of vaccines against Lassa fever and Middle East Respiratory Syndrome (MERS), which are also caused by a coronavirus.
Dr. J. Joseph Kim, Inovio’s CEO, said, “We’re extremely honored to expand our partnership with CEPI to tackle this new threat to global public health. Our DNA medicine platform represents the best modern day approach to combatting emerging pandemics.
“We have already demonstrated positive clinical outcomes with our vaccine against MERS-CoV, another coronavirus,” Kim said.
Kim said following the Zika viral infection outbreak about five years ago, Inovio and its partners developed a vaccine that went from bench to human testing in just seven months, which he said was the fastest vaccine development on record in recent decades.
“We believe we can further improve upon this accelerated timeline to meet the current challenge of the emerging Chinese coronavirus,” Kim said.
Gilead Assessing Potential Use Of Ebola Drug As China Virus Treatment
Gilead Sciences Inc said on Thursday it was assessing whether its experimental Ebola treatment could be used against the new coronavirus that has sickened hundreds of people in China and led to at least 18 deaths.
“Gilead is in active discussions with researchers and clinicians in the United States and China regarding the ongoing Wuhan coronavirus outbreak and the potential use of remdesivir as an investigational treatment,” a company spokesman said in an emailed statement.
Dr. Anthony Fauci, director of the U.S. National Institute of Allergy and Infectious Diseases (NIAID) told Reuters his agency was working with Gilead to test the company’s antiviral drug in people infected with the new coronavirus.
NIAID had previously tested remdesivir in patients with Ebola and found it to be ineffective. Fauci said there is some indication that it may work better against this new virus from China.
Coronavirus infections can lead to respiratory illnesses, some of which can be severe and deadly such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). They can also be much milder. The common cold is a strain of coronavirus.
While it is not yet known if the drug will be effective against the new strain of coronavirus that emerged from the central Chinese city of Wuhan, testing in animals had shown activity against the SARS coronavirus.
China has put millions of people on lockdown in two cities at the epicenter of the outbreak as authorities around the world work to prevent its spread globally.
Updated: 2-25-2020
First U.S. Testing Begins for Potential Coronavirus Treatment
NIH-sponsored study of experimental Gilead drug includes American patient evacuated from Diamond Princess cruise.
Researchers have begun the first study in the U.S. of an experimental drug aimed at treating the novel coronavirus, the National Institutes of Health said Tuesday.
Researchers at the University of Nebraska have started testing an experimental antiviral drug from Gilead Sciences Inc.,the NIH said. The first U.S. study subject is an American who is among 13 passengers who were quarantined on the Diamond Princess cruise ship and transported recently to the University of Nebraska Medical Center on Feb. 17 for further isolation and possible treatment, the NIH said. Eleven of those 13 passengers are infected by the virus, according to the Centers for Disease Control and Prevention.
The NIH-sponsored study is part of public health officials’ race to determine quickly whether the Gilead drug, called remdesivir, is effective in treating Covid-19, the illness caused by the new coronavirus. The study is designed to enroll up to nearly 400 patients globally, who will be randomly selected to receive remdesivir or a placebo.
While the illness has led to thousands of fatalities world-wide, most people who become infected experience flu-like symptoms such as fever and cough before recovering on their own, health officials have said.
The U.S. remdesivir study is scheduled to be completed in April 2023, but researchers could have preliminary results within a year, said Andre Kalil, a professor of internal medicine at the University of Nebraska who is leading the study for the NIH.
The timing is dependent on how quickly the outbreak is contained. “In the worst case scenario, if the outbreak just goes crazy and things get way worse than predicted, then the 400 patients will be enrolled really fast, in a few months,” Dr. Kalil said.
“This is probably the most rapid trial initiation we’ve seen in American history, because the trial was just designed a few weeks ago at the NIH, and we were able to get started right away,” he said.
The NIH is also expected by the end of April to start testing in healthy volunteers an experimental coronavirus vaccine developed by Moderna Inc. The Cambridge, Mass., drugmaker said Monday it had shipped its first batch of the experimental vaccine. A vaccine could prevent new infections but wouldn’t treat confirmed cases.
To be included in the remdesivir study, patients will have to have more advanced disease, including pneumonia-like symptoms, said Dr. Kalil. That would exclude the 80% or more of infected patients who suffer only mild symptoms similar to those of the flu or common cold.
The NIH is aiming to enroll patients in countries around the world where patients are infected, such as in Japan and Italy, said Dr. Kalil. The study will also allow for new experimental treatments to be introduced if and when they become available, he said.
Earlier this month, Chinese researchers began studying remdesivir in two clinical trials, but have struggled to enroll patients quickly because of the way the study is designed, The Wall Street Journal has reported. The Chinese studies will enroll patients with severe disease who were infected within just 12 days before starting the trial, and patients with moderate disease infected within the prior eight days, and who haven’t tried other medications. That criteria rules out most candidates for the studies, the Journal reported.
The NIH said it designed the U.S. study after taking into account the design of the Chinese studies.
Gilead is supplying the trial with remdesivir at no cost and is providing input on the study’s design and conduct, a company spokeswoman said.
Enrollment in the Chinese studies is also slowing down because the rate of newly infected patients has slowed in the country, said Bruce Aylward, head of an international team of scientists sent by the World Health Organization to China, on a conference call with reporters on Tuesday.
Gilead’s remdesivir has shown promise in animal studies and lab experiments as a treatment for Middle East respiratory syndrome, or MERS, and severe acute respiratory syndrome, or SARS, which are caused by other coronaviruses.
In late January, remdesivir was given as emergency treatment to a 35-year-old man Washington state infected with the new coronavirus. His condition improved rapidly after receiving the drug, and he was later discharged from the hospital.
These preliminary data for the drug are encouraging, scientists say, but have to be confirmed in controlled clinical studies before remdesivir can be approved for general use. “We don’t know if the medication will have the same effect in human beings [as in animals], and that’s why we need to test in this randomized trial,” said Dr. Kalil, the NIH study’s lead researcher.
Gilead, based in Foster City, Calif., has been ramping up manufacturing of the experimental drug to supply clinical trials and prepare for wider demand if the medicines proves effective.
Gilead’s share price is up 7.9% this year based on the company’s efforts to develop a treatment for the novel coronavirus, which, according to the NIH, has infected more than 79,000 people globally and killed about 2,600 people in just a few months. The vast majority of infections and fatalities have occurred in China, where the virus originated.
Drug company Regeneron is in the early stages of work on a potential treatment for this coronavirus. The company previously developed a similar treatment for Ebola.
Do Disinfectants Kill The Coronavirus?
Yes, they can. The CDC suggests that anyone exposed to an infected patient clean all “high-touch” surfaces, such as counters, tabletops, doorknobs, bathroom fixtures, toilets, phones, keyboards, tablets and bedside tables.
Cleaning agents can include a household disinfectant with a label that says “EPA-approved,” according to the CDC. A homemade version can be made, using one tablespoon of bleach to one quart of water.
Prognosis
China Warns Virus Spread Increasing, More Cases: Virus Update
The novel coronavirus spread further and killed more people as Canada confirmed its first case and China reported an increase in fatalities and infections.
The virus has killed at least 56 people in China, and President Xi Jinping on Saturday ordered a faster response, sending teams into hard-hit areas to push local officials to strengthen prevention and containment.
More than 2,000 cases have been reported in 15 countries and territories. South Korea on Sunday reported another infection, while Pakistan denied it has a confirmed case.
Crypto And Blockchain Firms Pitch In To Help Coronavirus Victims
Some blockchain and cryptocurrency firms have pledged to help victims of the coronavirus in Wuhan, China. Cryptocurrency exchange Binance pledged to donate 10 million Chinese yuan ($1.44 million) to the effort.
In a tweet on Jan. 25, Binance CEO Changpeng Zhao said that Binance made the pledge but did not make any announcements after a Twitter user tagged him in a news article about cryptocurrency donations being accepted for the cause:
“For #Wuhan, not realistic to do crypto to end beneficiaries. Binance pledged 10m RMB ($1.5m USD) to help #coronavirus victims. We didn’t make any announcements. But [Binance Charity Foundation] BCF/Binance team has been busy for the last few days. Still need help to arrange logistics locally.”
According to a Jan. 25 WeChat post by blockchain marketing service firm Krypital, the firm also launched a charity donation effort to acquire medical supplies for Wuhan coronavirus victims.
Krypital also announced that it will create a blockchain-based donation system that allows for greater transparency and efficiency. The firm accepts Tether (USDT) on the Ethereum blockchain.
The company is also recruiting volunteers for group administration, material purchase, sorting and transportation management, media announcements and graphic designers.
China Warns Virus Spread Increasing, More Cases: Virus Update
The novel coronavirus spread further and killed more people as Canada confirmed its first case and China reported an increase in fatalities and infections.
The virus has killed at least 56 people in China, and President Xi Jinping on Saturday ordered a faster response, sending teams into hard-hit areas to push local officials to strengthen prevention and containment.
More than 2,000 cases have been reported in 15 countries and territories. South Korea on Sunday reported another infection, while Pakistan denied it has a confirmed case.
- About 1,975 Cases In China, At Least 56 Deaths: Tracking The Outbreak
- Track Business And Travel Disruptions
- QuickTake: Learn More About The Virus
Here Are The Latest Developments:
China CDC Advises Extending Holiday (5:01 p.m. HKT)
Gao Fu, head of the Chinese Center for Disease Control and Prevention, told reporters that the agency is advising that the Lunar New Year holiday ending Jan. 30 be extended due to the virus. The decision will depend on how the situation develops, he said.
Beijing will lengthen the winter break for schools from kindergarten to college, People’s Daily reported, citing the city’s education bureau.
Hong Kong Confirms Sixth Virus Patient (4:50 p.m. HKT)
A Hong Kong health official confirmed the sixth case of the coronavirus in the city.
The South China Morning Post earlier reported that the man had been to Wuhan and arrived in Hong Kong by high-speed rail. He will undergo more tests. It was not known when he returned from China, the newspaper said.
Protest Over Proposed Quarantine Center (4:15 p.m. HKT)
Government plans to use a newly built, unoccupied public estate in the New Territories district of Fanling for possible patients under quarantine and medical staff drew an angry response from residents and district councillors.
A couple dozen masked people barricaded a road in Fanling in protest at the government proposal to use Fai Ming Estate as an emergency medical facility. Some of the protesters said the building is too close to their homes, while others complained that approved applicants would lose their flats in the estate.
China Says Pathogen’s Transmission Is Increasing (4:25 p.m. HKT)
Chinese authorities on Sunday told reporters the virus isn’t yet under control despite aggressive steps by authorities to limit movement for millions of people who live in cities near the center of the outbreak. Officials said information on the new virus is limited even though the pathogen was identified relatively quickly, and its transmission is increasing.
The government said it will hold daily press briefings on the situation.
Thailand, California Report Cases Of Infection (4:01 p.m. HKT)
Thailand’s director of communicable diseases said at a coronavirus meeting that the nation has eight confirmed cases of the illness. Those infected all came from outside the country, and there has been no local transmission so far.
California reported its first confirmed case, according to a statement from the Orange County Health Care Agency’s Communicable Disease Control Division. The person, who traveled from Wuhan, is in isolation in a local hospital and in good condition, it said.
China Bans Wildlife Trade Across The Country (2:36 p.m. HKT)
China banned the shipping and sale of wild animals starting Sunday and said it will quarantine breeding sites. Trade will be forbidden in markets, supermarkets, restaurants and online, the market supervision administration, agricultural ministry and forestry bureau said in a statement.
It also warned people against consuming wild animals. The new coronavirus was first found in people who shopped or worked at a so-called wet market in the central city of Wuhan, where live animals were sold.
China has tightened controls on the sale of exotic animals, considered nourishing in some parts of the country, though some are still sold surreptitiously.
AbbVie Anti-Hiv Drugs In China Treatment Plan (12:13 p.m. HKT)
A clinical trial is underway using anti-HIV drugs ritonavir and lopinavir to treat cases of the new coronavirus, according to an article published in the Lancet medical journal Friday. Beijing’s municipal health commission said on Sunday the drugs made by AbbVie Inc. are part of the National Health Commission’s latest treatment plan, and its hospitals have supplies of the medicine if needed.
On Sunday, China’s Global Times tweeted that the nation’s Center for Disease Control and Prevention will start developing a vaccine.
U.S. Charters Flight To Evacuate Citizens (11:34 a.m. HKT)
The U.S. government is making plans for its Wuhan consulate to arrange a charter flight to evacuate its citizens on Tuesday, AP reported.
The plane can seat 230 people and the consulate is approaching Americans to offer to evacuate them with costs borne by those who accept it, Dow Jones said earlier. The State Department told Reuters that there will be “limited capacity,” according to a tweet by one of its journalists.
Wuhan Doctor Who Died Wasn’t On Front Lines (11:20 a.m. HKT)
A 62-year-old doctor who died from the virus on Saturday in Hubei province contracted the virus from another elderly member of his choir, and was not on the front line as some media had reported, according to a person familiar with the situation. He was retired but had been rehired on a part-time basis by another hospital, the person said.
Hong Kong Theme Parks Shut (9:30 a.m. HKT)
Hong Kong theme parks including Disneyland and Ocean Park said they will be closed until further notice after the government stepped up prevention measures to curb the spread of the virus. Hong Kong on Saturday raised its response level to the coronavirus to “emergency” and said it will cancel its largest marathon scheduled to take place early next month.
Taiwan Fines Victim (8:30 a.m. HKT)
A man was fined NT$300,000 ($10,000) after he failed to report symptoms of a respiratory infection after traveling to Wuhan, the official Central News Agency reported, citing health authorities. He visited a nightclub without wearing a face mask the day after he returned to Taiwan, and a female employee later developed symptoms including a cough, according to the report. She has since been quarantined.
Pakistan Denies Infection (Sunday, 6 a.m. HKT)
A top Pakistan health official said there are no confirmed cases of the novel coronavirus in the country after reports on Saturday of an infection.
A patient kept in isolation in a hospital is improving and has no signs of a severe acute respiratory infection, State Minister of Health Zafar Mirza said in a post on Twitter on Saturday. He was earlier reported as saying that Pakistan “lacks the facility” to detect the virus.
Canada confirms first case (5:45 p.m. EST)
Canada reported its first coronavirus case, a man in his 50s who fell ill in Toronto days after returning from China, Ontario Chief Medical Officer of Health David Williams said Saturday.
The man arrived from Wuhan via Guangzhou on Jan. 22 and went into a hospital the following day after feeling ill. On Saturday, the province’s public health lab confirmed the case as a presumptive positive case. Williams said Toronto public health authorities are in touch with federal officials to help determine the exposure to other individuals on the flights.
Britain Advises Against Travel To Hubei (10:35 p.m. GMT)
The U.K. has advised against travel to China’s Hubei province, epicenter of the global outbreak, and is urging British citizens in the area to get out if they are able.
The British Foreign and Commonwealth Office said citizens should comply with any additional screening measures set up by local Chinese authorities. The U.K. has since Jan. 22 monitored flights arriving in the country from Wuhan, and has providing screening where needed, the government said.
3 Chinese Doctors Are Infected (4:35 a.m. HKT)
Three Chinese doctors, including two who visited Wuhan for business, are infected with the new coronavirus in Beijing, the People’s Daily reported in a tweet, without saying how it got the information.
The three physicians along with people they came into contact are in quarantine, according to the Daily, official newspaper of China’s Communist Party. All three are in stable condition, CCTV reported.
China Blocks Outbound Tours (10:40 p.m. HKT)
China banned all outgoing overseas group tours starting on Jan. 27, and suspended domestic group tours as of Jan. 24, CCTV reported. Beijing also will prohibit buses from entering and leaving the city from Jan. 26
The Ministry of Culture and Tourism had ordered travel agencies and tourism companies to stop selling tour packages, according to a document seen by Bloomberg. The ban coincides with the start of the Lunar New Year holiday, when millions of Chinese travel across the country and abroad.
Australian Cases Rise(6:45 p.m. HKT)
Australia reported three cases of coronavirus in the state of New South Wales, with the men diagnosed after travelling to China. Two were in Wuhan, the epicenter of the virus, while another man had contact with an individual confirmed to have the virus, a statement from New South Wales Health said. The country reported its first case earlier on Saturday in Melbourne.
China Urges Calm Over Virus During ‘Critical Period’
Wuhan mayor expects another roughly 1,000 confirmed coronavirus infection cases; Hong Kong to deny entry to people who have visited Hubei during the past two weeks.
Chinese public-health officials urged calm on Sunday even as they warned that the dangerous new virus at the heart of a fast-spreading outbreak is growing more contagious, piling more pressure on an already strained containment effort.
“We are now in a critical period of prevention and control,” Ma Xiaowei, head of China’s cabinet-level National Health Commission, said at a press briefing in Beijing on Sunday to deal with the unnamed coronavirus that has infected more than 2,000 people and killed at least 56, the vast majority of them in central China’s Hubei province.
Zhou Xianwang, the mayor of Wuhan, the epicenter of the outbreak, said at a press briefing late Sunday that he expects that experts will confirm another roughly 1,000 of the suspected infection cases under monitoring. The vast majority of the confirmed cases in mainland China so far are in and around Wuhan. Mr. Zhou said more than five million people have left Wuhan, leaving about nine million. Many residents hail from around the region and normally leave town for Lunar New Year, and it isn’t known how many specifically fled because of the virus.
The central government also sent the message on Sunday to the rest of the country that it is taking over from local officials in Hubei, whose sluggish reaction to the outbreak has drawn criticism.
Hong Kong’s local government on Sunday said it would deny entry to people who have visited Hubei during the past two weeks in an effort to restrict the spread of the virus.
The city has confirmed six cases of the virus in Hong Kong, all from patients who either lived in or recently visited Wuhan in Hubei.
The Hong Kong government said the ban will reduce the chance of infected people arriving in the city.
State media reported that Premier Li Keqiang has been put in charge of the Communist Party’s new “leading small group” of senior officials that is directing response to the virus nationwide. China’s official Xinhua News Agency reported that the group plans to dispatch a team to Hubei to lead efforts on the ground.
In an unprecedented move, officials have locked down several cities in Hubei, including Wuhan. But the cities weren’t sealed off until after millions of residents had scattered to visit family for Lunar New Year.
The virus has quickly spread to more than a dozen countries in Europe, Australia and Asia, prompting global concern.
On Saturday, health authorities in southern California’s Orange County said a traveler from Wuhan was carrying the virus, the third confirmed case in the U.S. In Hong Kong, a health official confirmed on Sunday the city’s sixth case of the new coronavirus, a 47-year-old man who arrived in Hong Kong on Thursday after visiting a market in Wuhan, though not the one at the center of the outbreak. Canada reported its first case, in Toronto.
In Beijing, officials from a number of government ministries sought to project a sense of control at a press conference, but acknowledged shortfalls in supplies and gaps in knowledge about the nature of the coronavirus.
Gao Fu, director of the Chinese Center for Disease Control and Prevention, reflected the seriousness of the epidemic and the exhaustion felt by the country’s health-care workers as he called for restraint during a frenzied question-and-answer session with reporters.
“Calm, calm down,” the Oxford University-trained immunologist said in English, holding up his hands, before switching to Mandarin: “The virus has become rampant. Everyone here is anxious.”
Authorities highlighted the preventive measures they were implementing. Li Bin, deputy director of China’s National Health Commission, said officials were monitoring the health of migrant workers who had returned home for the holidays, especially those who traveled from Wuhan.
The National Health Commission said it encouraged people to scan their neighborhoods for anyone who might have come from or spent time in Wuhan.
Around the country, workers and community members were dispatched to go door to door asking people if they had been to Wuhan or Hubei province.
“If you have been to Wuhan recently, or have been in contact with and know about people who have come back from Wuhan, please inform the community,” said one poster at an apartment building in Chengdu.
In the southern provinces of Jiangxi and Guangdong, authorities have required everyone to wear face masks in public.
In Wuhan, the center of the epidemic, the medical system continued to struggle to keep pace with the crisis, despite government injections of supplies, staff and money.
The city’s medical workers were in dire need of more protective suits, according to Wang Jiangping, vice minister of industry and information technology. Wuhan is going through 100,000 single-use suits a day, he said. China’s factories, operating at 40% capacity because of the holiday, can produce only 30,000 a day.
He said the government had purchased enough suits from abroad to serve Wuhan’s needs for two more days.
The central government planned to send an additional 1,600 medical personnel to Hubei province within two days, officials from the health commission said.
The Wuhan government had requisitioned hundreds of buses and thousands of taxis to transport anxious patients and harried medical staff around the city, according to Liu Xiaoming, a vice minister of transportation. City authorities had earlier suspended public transportation and nonemergency car traffic in a bid to keep sick residents from infecting others, leading to fears that hospital staff might not be able to get to work.
The CDC’s Mr. Gao said the virus wasn’t showing signs of mutating into a more deadly form. “As it transmits from human to human, it would evolve and mutate based on our past knowledge,” he said.
Mr. Gao said a vaccine would be available soon, though he didn’t give a timeline for its availability.
There is no known cure for the new virus, which causes pneumonia symptoms and is particularly dangerous to the elderly and those with compromised immune systems. It is similar to the coronavirus that caused an outbreak of severe acute respiratory syndrome, or SARS, in 2002 and 2003, which killed nearly 800 people after emerging in southern China.
Unlike SARS, which caused a high fever, initial symptoms associated with the new virus are milder, making it harder to detect, Mr. Gao said.
In a search for treatments, Beijing city health authorities have begun experimenting with antiretroviral medication lopinavir, used to treat HIV, Xinhua reported on Sunday.
Beijing’s education committee said on Sunday that all schools in the city, from kindergartens to universities, would be required to delay the spring semester for an unspecified period, state media reported.
Shanghai’s municipal education and health authorities said primary and secondary schools wouldn’t be permitted to hold any teaching or group activities before Feb. 17 and should cancel holiday-return activities. All day-care facilities would be closed until the end of February.
Officials in Suzhou, a city near Shanghai that is a magnet of foreign investment, instructed companies and businesses not to begin operations again before Feb. 9.
Also on Sunday, local officials on China’s southern coast appeared to waver over whether to be the first outside the Wuhan area to order the lockdown of a city.
China Central Television reported in the morning that Shantou, a port city of about five million people more than 600 miles south of Wuhan, planned to suspend all inbound car, boat and pedestrian traffic at midnight. Public transportation, including ferries and taxis, would also be suspended, the state broadcaster said.
In a sign of how uncertain local authorities are about how to respond to the crisis, officials removed the lockdown announcement from Shantou’s online notice board and replaced it with a message saying transportation would be allowed to carry on as usual after being disinfected.
By the time the second notice was posted, people visiting relatives in Shantou for the Lunar New Year holiday had rushed to get out of town before the deadline. Residents had stocked up on staples, exhausting supplies of rice in supermarkets around the city.
“We adjusted the decision according to our assessment and feedback from the public,” said a person who answered the phone at Shantou’s outbreak-response command center. “Don’t worry, and no need to panic.”
Travel plummeted on Saturday, the first day of the Lunar New Year, compared with the same period the year before, according to Mr. Liu, the transportation official. Railway and air traffic each dropped by more than 40% while road traffic fell by a quarter.
Mr. Liu urged local officials not to set up on their own quarantine barriers that might block the shipments of medical supplies.
Images circulating on Chinese social media sites, which the Journal couldn’t verify, showed blockaded roadways where men were holding clubs and sticks, guarding village entrances and posting signs saying outsiders weren’t welcome.
Virus Sparks Soul-Searching Over China’s Wild Animal Trade

Beijing faces uncomfortable questions over its failure to clean up wildlife trade and public calls for a permanent ban on wild meat.
It didn’t take long to identify the suspected source of a deadly coronavirus outbreak in the Chinese city of Wuhan: a cluster of vendors in a downtown market offering carcasses and live specimens of dozens of wild animals—from bamboo rats to ostriches, baby crocodiles and hedgehogs.
The Huanan food market, a scruffy complex of 1,000 stalls spread over an area the size of nine football fields, is the largest of its kind in central China, mostly supplying seafood to Wuhan’s residents and restaurants. It is typical of the wet markets where most people in this country buy their food.
Like many such markets, it also sold wild animals enjoyed as culinary delicacies or used as traditional medicine—an ancient trade Beijing has continued to allow despite warnings that it caused a deadly coronavirus outbreak almost two decades ago, and could trigger another global epidemic.
After giving Huanan market an all-clear during inspections late last year, city officials have now closed it. When Wall Street Journal reporters visited this past week, it was cordoned off by police tape and stall holders were lining up in the rain to receive compensation and Lunar New Year handouts.
Racing to contain the outbreak, China’s national authorities have locked down Wuhan and several other cities in Hubei province, of which Wuhan is the capital. On Sunday, they imposed a temporary nationwide ban on the trade of wild animals and quarantined all wildlife breeding centers.
Even so, Beijing now faces uncomfortable questions over its failure to clean up the wildlife trade in recent years. It is also confronting unusual public calls in China for a permanent ban on wild meat, something it has been reluctant to impose for fear of angering its relatively wealthy aficionados.
China’s president, Xi Jinping, has spoken about the country’s ability to show leadership on global issues, such as public health. And his response to the current crisis is likely to be seen as an important test, health experts and political analysts say.
“This incident should be used as an opportunity to rectify the chaos” in China’s wildlife trade, said a petition published on Thursday by 19 prominent Chinese scientists, including a former head of Peking University.
Medical researchers have determined that the outbreak of severe acute respiratory syndrome, or SARS, that started in 2002 originated in bats and spread to humans via palm civets—cat-sized mammals that look a bit like weasels—sold in Chinese food markets.
Studies have shown that SARS-type coronaviruses reside naturally in bats but can easily jump to other hosts, mutating along the way, especially in markets where species, including humans, mingle.
Although Chinese authorities have yet to identify the precise origin of the current outbreak, a study released on Thursday by the Wuhan Institute of Virology, based on patient samples, found a 96% genetic match with a bat coronavirus.
Another Chinese study suggested snakes sold in the market were the source, although other scientists think it less likely the virus jumped between reptiles and mammals.
“This is a wildlife-origin virus—it’s pretty clear,” said Peter Daszak, president of EcoHealth Alliance, a U.S.-based nonprofit organization that has been studying the origins of SARS and related viruses in China for 15 years.
“Probably bats are the origin from looking at the virus itself, and it got from bats into people in the wildlife market,” he said. “This is absolutely déjà vu all over again from SARS.”
China is a hot spot for such outbreaks because it combines large bat populations with densely populated rural areas and a long tradition of eating wildlife, especially in the southern provinces of Guangdong and Guangxi.
“Why do I eat it? It is delicious,” said Terry Gao, a 30-year-old businessman from Guangxi, where he usually eats wild meat. He said he had a particular taste for civets.
“It is really hard to describe,” he said. “Like how lamb has that special taste, civets are the same. Just the flavor of the meat itself. You don’t need to cook it in any special way: Once you taste it, you’ll know it’s civet.”
He said he had long known of the health risks, and would avoid eating wild meat during the current outbreak but attributed the problem to poor regulation rather than consumer demand.
Since SARS, China has vastly improved its capacity to respond to disease outbreaks, health experts say. It has also improved hygiene at wet markets, and sought to encourage licensed trade in wild animals bred on farms where they must undergo sanitary checks.
And yet regulation of wildlife farms and markets has been lax. As a result, an underground trade has thrived, with restaurants often commissioning wild meat—including endangered species—from hunters via middlemen, researchers and wildlife activists say.
Online trading has also made it easier to source and distribute wild meat across China, and to import creatures such as pangolins from other countries, exacerbating the risk of infections spreading over longer distances.
Two men from the eastern province of Jiangxi, known as the Huanong Brothers, have even become video-streaming stars in China by posting clips from the farm where they breed bamboo rats for their meat, as well as from their regular trips to hunt for wild animals.
“If China doesn’t take action on this now, I fear this is just going to happen again,” said Zhou Jinfeng, head of the nongovernmental China Biodiversity Conservation and Green Development Foundation.
Mr. Zhou, who has filed official complaints about wildlife markets all around China, estimated that there were hundreds in the country, with at least one in every major city like Wuhan, and more still online.
“The authorities just don’t take any notice,” he said. “They think it’s good for the local economy.”
China banned all wildlife trade in 2003, when Hong Kong researchers first identified civets as a potential source of SARS, but it lifted the ban later that year on 54 species—including civets—that it said could be bred in licensed farms, subject to sanitation checks.
Guangdong province banned the breeding and sale of civets in 2004, but they continued to be traded there and in other provinces. On Thursday, Guangdong imposed a total ban on wild-animal trading.
The central government, however, was slower to respond. First the agriculture ministry ordered a halt Friday to the trade only of wild animals that can carry the coronavirus, which it said included badgers and bamboo rats. Two days later, the State Administration of Market Regulation announced a ban on all wildlife trade but said it would end once the outbreak was over.
The Communist Party’s Central Commission for Discipline Inspection, usually focused on fighting corruption, also made an unusual appeal for people to stop eating wild meat in a posting on its website in recent days.
“We must respect the laws of nature and promote scientific and healthy eating habits,” it said.
The market at the epicenter of the current outbreak, officially known as the Wuhan Huanan Seafood Wholesale market, was home to vendors selling a wide range of wild meat.
One of them, called Dazhong Livestock and Game, boasted that it could provide more than 100 wild animals, freshly slaughtered or flash frozen, on site or via home delivery, according to a price list published online.
Among the most expensive items were a live ostrich for 4,000 yuan (about $580) and a small live deer for 6,000 yuan. The list also included baby crocodiles, wolves and hedgehogs. The owner couldn’t be reached for comment.
The Wuhan Market Regulation Administration inspected the market in November and December but found nothing wrong, according to documents published on its website. It didn’t respond to a request for comment.
In September, local officials also inspected some eight stalls selling wild animals and checked their business licenses but found nothing illegal, according to the website of a newspaper run by Wuhan’s Communist Party committee.
In a rare admission for a senior Chinese official, Wuhan’s mayor, Zhou Xianwang, told the official Xinhua News Agency that local authorities had failed to properly regulate the market—one of 400 in the city.
The first signs of the outbreak came on Dec. 29, when four workers at the market were admitted to a Wuhan hospital with pneumonia.
The hospital alerted the local center for disease control, and Wuhan authorities closed the market on Jan. 1. Health officials took specimens from the site and found evidence of the virus in 33 out of 585 samples, according to the Chinese Center for Disease Control and Prevention, or CCDC.
The virus had been found not just in people’s bodies, but on wild meat stalls, Gao Fu, the CCDC director told Chinese state television on Thursday.
“We must thus call on everyone not to eat wild animals,” he said. “It is only a matter of time to find out which is the specific animal.”
Coronavirus Is Driving Sales Of Face Masks, A Game Called Plague, And An ‘I Survived Coronavirus 2020’ T-shirt
Demand is high for face masks and a plague-themed strategy game.
Welcome to the coronavirus marketplace.
Amid rising infection cases and deaths from the new coronavirus, protective gear like face masks and at least one game related to disease outbreak have surged in sales. Meanwhile, some sellers have sought to capitalize on the growing alarm.
Cases of the new coronavirus were first detected in Wuhan, China and have since been reported in nearly a dozen countries, including the U.S. The virus has killed at least 26 people and infected more than 900, mostly in China, according to an NBC News data analysis. The Centers for Disease Control and Prevention confirmed Friday that a second U.S. case had been detected, this time in Chicago.
Face Masks Are Flying Off Shelves In China
Patients with this strain of coronavirus have reported having a respiratory illness ranging in severity, with symptoms including fever, cough and shortness of breath, the CDC says. While the federal agency said it doesn’t yet know how easily this particular coronavirus spreads — noting that some viruses are more contagious than others — many patients in Wuhan had apparently had contact with a live animal and seafood market, and the infection later appeared to be spreading person-to-person among patients with no exposure to these markets.
“When person-to-person spread has occurred with MERS and SARS, it is thought to have happened via respiratory droplets produced when an infected person coughs or sneezes, similar to how influenza and other respiratory pathogens spread,” the CDC says on its website. “Spread of SARS and MERS between people has generally occurred between close contacts.”
Accordingly, face masks have flown off shelves, reportedly prompting factories to reopen ahead of China’s Lunar New Year celebrations. Cao Jun, the general manager for Lanhine, a mask manufacturer in China, suggested to Reuters this week that a nationwide mask shortage was “much, much more severe than what the public knows.” All told, client demand has reached a cumulative 200 million masks a day, he said — dwarfing Lanhine’s typical daily output of 400,000.
“At the moment, we have 20-plus people in the factory, working 24 hours. We’re offering them quadruple their wages per day,” Cao told Reuters. “We aim to ramp up output on Jan. 27 and be at full capacity on Feb. 1, when we’d have nearly 200 workers.”
Alibaba-owned BABA, retailer Taobao sold some 80 million face masks on Monday and Tuesday, the BBC reported, and cautioned sellers against hiking up prices after reports of people flipping masks for profit.
A spokesman for Honeywell, a leading manufacturer of protective face masks, told MarketWatch the company was experiencing “a surge in demand” in North America, Europe and China. He declined to disclose specific sales figures.
“We are increasing production at multiple facilities globally, and we are fulfilling all current orders,” the spokesman said. “In China, our products are available from JD.com and TMALL.com, and in the U.S. and Europe, they can be found at a number of retailers, including Amazon.”
Meanwhile, shares of companies that manufacture rubber gloves have jumped as the coronavirus spreads. Stock for the Malaysian manufacturer Top Glove rose nearly 14% over two days, the Financial Times reported Wednesday, while Malaysian latex glove makers Supermax Corp. and Kossan Rubber Industries increased Wednesday by a respective 8% and 6%.
One eBay Seller Is Hawking A ‘Coronavirus Protection Kit’
A number of eBay sellers in recent days have also listed respirator masks, air purifiers and disposable fluid-resistant jackets, invoking “coronavirus” in their listing titles. A $21.99 “coronavirus virus protection kit,” which claims to offer “full body protection,” includes a 3M MMM, +0.23% mask, splash-resistant goggles, a disposable white coverall, medium and large pairs of nitrile gloves, antibacterial wipes, alcohol hand sanitizer and a clinical-waste biohazard bag.
A Virus-Themed Online Game Is Gaining Players
Consumers haven’t limited their virus-preparation measures to safety products. The strategy and simulation game Plague Inc., which has players “bring about the end of human history by evolving a deadly, global plague” as the world scrambles to defend itself, became China’s best-selling app on Wednesday, according to the BBC. The game sells on Apple’s App Store for $0.99.
In its eight years of existence, Plague Inc. has typically seen a increase in players during disease outbreaks “as people seek to find out more about how diseases spread and to understand the complexities of viral outbreaks,” the game’s U.K.-based developer, Ndemic Creations, said Friday in a statement posted to Twitter.
“We specifically designed the game to be realistic and informative, while not sensationalising serious real-world issues,” the statement read. “However, please remember that Plague Inc. is a game, not a scientific model and that the current coronavirus outbreak is a very real situation which is impacting a huge number of people.” The developer directed players to the World Health Organization’s resources on coronavirus.
T-shirts In Questionable Taste And A Timely NetFlix Series
Of course, however scant, there is online merchandise — including an “I Survived Coronavirus 2020” T-shirt ($16.49) on Etsy that’s described as a possible “instant favorite in every nurse’s wardrobe” and a “Corona Virus” shirt ($21.98) on Redbubble that’s styled with the AB InBev-owned BUD, Mexican lager brand’s logo. (AB InBev did not respond immediately to a request for comment.) A Redbubble spokeswoman said the “Corona Virus” shirt was removed from the site. “The Coronavirus-themed design in question is a violation of Redbubble’s community guidelines regarding sensitivity around works that deal with catastrophic events,” said spokeswoman Marissa Hermo. “We have removed the design and will continue to monitor for any other artwork that similarly violates these guidelines.”
Netflix inadvertently got in on the viral marketing, debuting its six-part docuseries “Pandemic: How to Prevent an Outbreak,” which profiles health-care professionals and their efforts to battle virus outbreaks, this week. Netflix did not immediately respond to a request for comment on the release timing, but executive producer Sheri Fink addressed speculation in a Twitter thread.
“At times like these, many people’s thoughts turn to the existential threat to humanity posed by viruses,” Fink said. “But across the world each day, people work fiercely to protect us. Pandemic follows them.”
“No, we didn’t know there would be a scary outbreak on Pandemic’s long-planned release date (please),” she added. “But this highlights the point: the risk never eases.”
Updated: 1-30-2020
Coronavirus Triggers Damage Control From Governments, Companies
India and the Philippines confirm first infections, as companies temporarily halt operations in China and Russia tightens its border.
Government officials and corporate executives around the world are scrambling to limit the damage from the fast-spreading coronavirus as Russia tightened its border with China and the U.S. announced plans for a second evacuation of the Chinese city at the center of the epidemic.
In response to the virus, companies including Tesla Inc. and IKEA were forced to temporarily halt operations in China.
The moves came as two more countries—India and the Philippines—confirmed their first infections, bringing the total number of affected countries to nearly 20, as the total number of confirmed cases approached 8,000.
India said a student from Wuhan University tested positive for the virus while visiting the southern state of Kerala and was in isolation at a local hospital. The Philippines said Thursday it recorded its first confirmed coronavirus case, a 38-year-old Chinese woman who arrived in the country on Jan. 21 from Wuhan.
Moscow, meanwhile, said that it will temporarily restrict passage through 16 road, rail and river checkpoints along its 2,670-mile-long border with China, starting Friday. Though Russia’s national carrier Aeroflot hasn’t stopped flying to China, smaller Russian airlines have canceled flights into China from the Far Eastern city of Vladivostok.
A number of countries pushed ahead with efforts to extract their citizens from central China.
The State Department on Thursday said it is planning a second evacuation flight from Wuhan, the central Chinese city where the newly identified coronavirus first emerged last month, offering hope for the hundreds of American citizens still believed to be in the city.
White House spokesman Hogan Gidley said the risk coronavirus poses to Americans remains low and that he wasn’t aware of government plans to cancel flights to and from China. He said the U.S. is taking “all the precautions necessary and will continue to do so.”
In a briefing on the administration’s efforts to combat the opioid epidemic, Assistant Secretary of Public Health Brett Giroir said it was under control in the U.S., noting there had been no person-to-person transmission of coronavirus in the country. “This is no cause for urgent panic,” he said.
The Indian government is seeking permission from Chinese authorities to operate two flights to repatriate citizens from Hubei province, of which Wuhan is the capital, and will quarantine them for 14 days.
In Japan, controversy erupted Wednesday after two people on a government-chartered evacuation flight from Wuhan to Tokyo refused to be tested for the new coronavirus. Some on social media wondered why the Japanese government didn’t quarantine evacuated citizens the way other countries had.
“This is unforgivable,” wrote one Twitter user. “No more charter flights!”
Prime Minister Shinzo Abe told Parliament Thursday that while the government had pushed for all 206 passengers on the Wednesday flight to be tested, it had no legal power to compel them. Three people on the flight tested positive for the virus, including two without symptoms, according to the health ministry. The two who refused testing didn’t show any symptoms and health-ministry staff drove them home in a regular car, health ministry official Takuma Kato said.
A second charter flight to evacuate Japanese citizens from Wuhan arrived in Tokyo on Thursday, and Mr. Kato said all 210 people who returned on that flight had agreed to be screened.
Meanwhile, immigration officials in Hong Kong scoured the city for visitors from Hubei, finding 15 on Wednesday night during searches of 110 hotels, according to Lam Shuk-yee, deputy secretary for security of the Chinese territory on Thursday.
Ms. Lam said 1,600 people from the province had been turned away at the Hong Kong border since the ban.
On the corporate front, big multinational companies moved to temporarily shut down their China operations as workers remained largely in place, with the Lunar New Year holiday extended through the end of the week and transportation links largely curtailed.
Tesla Chief Financial Officer Zach Kirkhorn said Wednesday that the company was halting production at its new Shanghai Gigafactory to comply with a local-government order to extend China’s Lunar New Year holiday, which Mr. Kirkhorn said could affect the company’s first-quarter performance.
All 30 IKEA outlets in mainland China were closed until further notice, the Swedish furniture giant said Thursday.
Air France —part of Air France-KLM—joined the list of airlines cutting service to China. The French carrier said it would suspend all scheduled flights to and from the mainland until Feb. 9 and would operate special flights starting Friday to and from Shanghai and Beijing using volunteer crew members to enable customers and employees to depart safely.
Italian authorities were holding 6,000 passengers and crew aboard a cruise ship docked at the port of Civitavecchia near Rome after a 54-year-old Chinese woman showed flulike symptoms, according to a spokesperson for Costa Crociere, the company operating the ship Costa Smeralda.
The woman and her male traveling companion, who showed no symptoms, were isolated in the ship’s hospital, the company said, while Italy’s health ministry said it was waiting for the results of tests for coronavirus.
China’s national women’s soccer team is being held in quarantine in a hotel in the Australian city of Brisbane until Feb. 5, health authorities for the northwestern state of Queensland said Thursday. The 32-member team had traveled to Australia to compete in a qualifying tournament for this summer’s Olympics in Tokyo.
The tournament was originally scheduled to be held in Wuhan but was moved to Sydney after the outbreak. The team had departed Wuhan Jan. 22, before the city was locked down, said the Chinese Football Association, which said it also planned to suspend soccer competitions nationwide starting Thursday.
Closer to the outbreak’s center, the education department of Wuhan’s home province of Hubei encouraged middle and primary schools to move classes online to ensure students keep up with their studies even with the Lunar New Year holidays extended indefinitely.
“The semester is delayed, but study shouldn’t be,” read a slogan included with the recommendation by the department, which separately encouraged local universities to move academic activities online.
The province also opened a new helpline on Thursday for people struggling with the psychological toll of the outbreak, China’s official Xinhua News Agency reported Thursday, citing local officials.
In Chongqing, a southwestern megacity that borders Hubei, pharmacies are now required to report the names of people who buy medication for symptoms like fever and cough, part of an effort to track people who might have coronavirus symptoms, state broadcaster China Central Television reported Thursday. The city of more than 30 million people had 165 confirmed cases of the coronavirus as of midnight Wednesday and is closely connected to Wuhan by road and rail.
In a sign of mounting pressures on medical staff in affected cities, the head of the infectious diseases division at Shanghai’s Huashan Hospital declared that all the doctors who had been treating coronavirus patients would be allowed to rest and would be replaced by doctors who were Chinese Communist Party members.
“We can’t bully those who are more obedient,” Zhang Wenhong said, describing the early responders as heroic. “So I’ve decided to change the shift. It will all be Party members from now on.”
In words tinged with exhaustion and frustration, Mr. Zhang, who is also the senior party leader of his division, said Communist Party members needed to live up to their vows to serve the people. “I don’t care whether or not you’re willing, you’re all going to step up,” he said.
Updated: 1-30-2020
WHO Declares Coronavirus Outbreak A Global Public Health Emergency
The designation comes as the first person-to-person transmission of the virus is reported in the U.S.
The World Health Organization declared the coronavirus outbreak a public-health emergency of international concern Thursday as the first person-to-person transmission of the virus was reported in the U.S.
The WHO designation, pointing to an increase in the number of cases, indicates that international public-health authorities now consider the respiratory virus a significant threat beyond China, where it originated last month. The move could further heighten the global response to the outbreak.
The agency made the declaration after a meeting of its emergency committee, which declined to do so last week. Since then, China, other governments and multinational businesses have taken emergency steps to limit the virus’s spread, including halting some travel to China.
In the U.S., a sixth person tested positive for the infection in the first case of human-to-human transmission. The patient is the husband of a Chicago woman infected with the virus whose case was reported last week. She had recently traveled to Wuhan, the central Chinese city where the coronavirus first emerged last month.
The U.S. Centers for Disease Control and Prevention and state officials emphasized that the overall risk for people in the U.S. and in Illinois remains low. “This person-to-person spread was between two very close contacts, a wife and husband,” said Ngozi Ezike, the director of the Illinois Department of Public Health. “It is not spreading in the wider community.”
Public-health authorities said the WHO designation helps mobilize resources to contain the virus’s spread. The WHO’s director-general can make recommendations to the international community, though they aren’t legally binding.
WHO Director-General Tedros Adhanom Ghebreyesus said he was confident in China’s capacity to control the outbreak, which has sickened more than 9,500 people and killed 213—up from 170 a day earlier—mostly in China’s Hubei province, which surrounds Wuhan.
“Let me be clear. This declaration is not a vote of no confidence in China,” Dr. Tedros said. “I have never in my life seen this kind of mobilization.”
The number of infection cases in China surpassed the global total for severe acute respiratory syndrome, though SARS killed nearly 800 people after emerging in southern China in late 2002 and into 2003.
Since it gained the power, in 2005, to declare an international emergency, WHO had applied the designation to just five prior situations. The first was in 2009 in response to the H1N1 swine flu, followed by polio in 2014 and the Ebola and Zika virus outbreaks in 2016. It declared a public emergency for another Ebola outbreak in 2019 and faced criticism for delaying that decision.
At least 18 other countries or territories have also reported a small number of coronavirus cases, with Finland, India and the Philippines now reporting cases in people who have traveled to Wuhan, according to WHO.
In response to the virus, Russia has tightened its border with China and the U.S. announced plans for a second evacuation of Americans from Wuhan. Companies including Tesla Inc. and IKEA temporarily halted operations in China.
The CDC has investigated 165 people in the U.S. for the virus, according to the numbers released Wednesday, and 68 have tested negative and been cleared. Over 90 cases are pending, and health authorities said that they expect additional cases.
The new Chicago patient lived with and was in consistent close contact with his wife. After returning to the U.S. on Jan. 13, she developed symptoms and was hospitalized in an isolated setting. Once her husband also started developing symptoms he was quickly taken to the hospital. The patient, who has underlying health issues, is in a stable condition, health authorities said.
“It is clear that this virus is highly transmittable, and this assumption is based on the rapid rate of spread of this infection in China,” said Eyal Leshem, director of the Institute for Travel and Tropical medicine at Sheba Medical Center in Israel.
“When there is a public-health uncertainty, you always want to slightly overreact to make sure that you don’t miss a critical issue,” Dr. Leshem said. “Once you learn a little bit more about the risk and the effective steps, then you can scale back.”
Health authorities believe the virus spread while the first patient in Chicago was symptomatic, rather than before.
Officials said the man, who is in his 60s, didn’t attend any mass gatherings. There are 21 people under investigation in Illinois for possible infection, Dr. Ezike said, and local and federal health authorities are working to monitor close contacts of the second Chicago patient.
Nancy Messonnier, director of the National Center for Immunization and Respiratory Diseases at the CDC, called the coronavirus “a very serious public-health situation.” She added: “We’re trying to spark a balance in our response right now.”
The CDC said that people who had recently traveled should be vigilant for symptoms and signs of the virus, which include fever, cough and shortness of breath.
President Trump during a speech in Michigan said the administration is working closely with China and sought to minimize fears about the virus in the U.S.
“We think we have it very well under control. We have very little problem in this country at this moment,” Mr. Trump said, adding that the handful of victims are recuperating. “We think it’s going to have a very good ending for us. That I can assure you.”
On Wednesday, Mr. Trump announced a task force to address the coronavirus, which he said had been meeting daily since Monday. The group is led by Health and Human Services Secretary Alex Azar.
Members of a House of Representatives panel briefed by federal medical officials Thursday said that traditional means of stopping infection are still the best guard against the virus’s spread.
Members of the subcommittee said federal officials appear to have the situation in hand and that there isn’t any need for a coronavirus “czar,” as was appointed during the Ebola virus epidemic in West Africa during the Obama administration.
Authorities in Russia, meanwhile, said they would temporarily restrict passage through 16 road, rail and river checkpoints along its 2,670-mile border with China. Though Russia’s national carrier Aeroflot hasn’t stopped flying to China smaller Russian airlines have canceled flights.
A number of countries have pushed ahead with efforts to extract their citizens from central China.
The State Department on Thursday said it is planning a second evacuation flight from Wuhan for the hundreds of American citizens still believed to be in the city.
The Indian government is seeking permission from Chinese authorities to operate two flights to repatriate citizens from Hubei province, and will quarantine them for 14 days.
Updated: 2-6-2020
Chinese Doctor Who Issued Early Warning On Virus Dies
Li Wenliang, a 33-year-old ophthalmologist based in Wuhan, had captivated the country and triggered an extraordinary outpouring of emotion as he ailed.
A Chinese doctor who became a folk hero after he was arrested for warning about the dangers of the deadly new virus now spreading around the world died on Friday after becoming infected with it.
Li Wenliang, a 33-year-old ophthalmologist based in Wuhan, the epicenter of the outbreak, had captivated the country and triggered an extraordinary outpouring of emotion as he ailed.
In social-media posts, many Chinese directed their frustration at government officials who many believe didn’t respond quickly enough despite clear evidence of the developing epidemic. Millions of people flocked to a live stream about Dr. Li that was run by local media outside the hospital where he was being treated.

“An all-out effort to save him was unsuccessful,” the hospital said. “We deeply grieve the loss.”
The World Health Organization on Thursday reported 28,285 confirmed cases globally, including more than 3,700 new ones. A total of 565 people have died, it said. Singapore, which has the second-largest number of cases outside China, reported two new infections, including one with no apparent link to China.
Chinese state media reported Thursday night that Dr. Li’s heart had stopped at around 9:30 p.m., and that he was immediately put on life support. The hospital where Dr. Li was being treated later said authorities were still fighting to keep him alive and then announced his death at 2:58 a.m. Friday.
After initial reports of Dr. Li’s death began circulating online late Thursday in China, including from the official social-media accounts of Communist Party publications, he was mourned and celebrated as a symbol of the public’s determination to find answers to still-unanswered questions about how officials first responded to the outbreak.
In an interview with the Communist Party-controlled Beijing Youth Daily newspaper in late January, Dr. Li recalled seeing reports in December of an unusual cluster of pneumonia cases linked to an animal market in Wuhan.
On Dec. 30, Dr. Li told the newspaper, he sent a message to former classmates on WeChat, a popular messaging app, warning them of new cases of severe acute respiratory syndrome, or SARS. He later corrected that, saying it was an unknown coronavirus.
Dr. Li was later interrogated by party disciplinary officials and hospital management, who accused him of spreading rumors and forced him to write a self-criticism, he told the newspaper.
“They told me not to publish any information about this online,” Dr. Li told the Beijing Youth Daily in late January. “Later, the epidemic started to spread noticeably. I’d personally been treating someone who was infected, and whose family got infected, and so then I got infected.”
In speaking out about the virus and about government efforts to silence him, Dr. Li drew comparisons to Jiang Yanyong, a surgeon who became a hero after blowing the whistle on Beijing’s efforts to cover up the extent of the SARS crisis in 2003. But people following the outbreak believe sickened medical staff in Wuhan number in the hundreds.
The Wuhan government doesn’t disclose the number of infected medical staff. To date, the most notable indication of infections among the medical community has come from Zhong Nanshan, another prominent doctor and a veteran of the 2003 SARS crisis, who disclosed in January that 14 medical staff had been infected by one patient.
Uncertainty hovered over Dr. Li’s status late Thursday night and early Friday morning, with elegies pouring in even as the hospital said he was still receiving emergency life support.
Some of the earliest reports of his death came from state media outlets’ social-media accounts, which sought to immediately venerate him, reflecting the confusion and contradictions that have dogged the Communist Party’s propaganda apparatus throughout the outbreak.
The English- and Chinese-language Twitter accounts of People’s Daily, the main Communist Party newspaper, were among the first to praise Dr. Li as a “whistleblower.” Within hours, the posts were deleted.
Instead, the Global Times, a nationalistic Communist Party newspaper, reported that Dr. Li was in critical condition several hours after his heart had reportedly stopped beating. He was then put on an artificial respirator.
Two editors at Chinese news media outlets said they received a notice to play down the death of Dr. Li by only reporting official announcements.
Meantime, thousands of users flooded Weibo demanding the Wuhan police offer a formal apology to Dr. Li. “Apologize to people all over the nation,” wrote one user.
“It happened so suddenly,” one doctor in Wuhan who knew Dr. Li said in a phone interview late Thursday night. “Humans are like ants sometimes. We are too small. It is such a hard blow to the frontline. Now our workmates are all very devastated.”
“Our hope is gone,” said another doctor in the city. “He was our hero.”
“We are very sad to hear of the loss of Dr. Li Wenliang, ” Mike Ryan, executive director of the World Health Organization’s Health Emergencies Program, said at a press conference in Geneva on Thursday. “We should celebrate his life and mourn his death with his colleagues.”
As confusion swirled about Dr. Li’s condition, more than 17 million people were watching the live stream for updates on Dr. Li’s status by 1:49 a.m. on Friday morning.
Under a post by the hospital about Dr. Li’s condition, a Weibo user wrote: “No sleep tonight!!! Waiting online for a miracle,” a comment that drew more than 334,000 likes.
Dr. Li, who was married with one child and another on the way, caught the dangerous new virus before Chinese authorities had stepped up its warnings about it. In the early days, he recalled, he didn’t wear any protective gear.
In several interviews with Chinese media while he was hospitalized, Dr. Li described how he was infected by a female patient who saw him for glaucoma in the second week of January. She had developed a fever and a CT scan showed an unknown virus in her lung. Two of her family members were also sick.
“It was such an obvious case of human-to-human transmission,” Dr. Li said, adding that he reported it to hospital officials right away.
A few days later, Dr. Li started coughing and his temperature rose. He booked a room in a hotel, worried that his child and his pregnant wife would be infected. A CT scan confirmed his fear; he was infected, and was hospitalized on Jan. 12, he wrote in a Weibo post last week, though he wasn’t counted as a confirmed case until Feb. 1, he said, nearly three weeks after he first showed symptoms.
The hospital put him under quarantine. Around the same time, he learned that his parents and some colleagues were infected as well.
“I was thinking then why the official announcement was still saying there had been no transmission between humans and of medical staff,” he wrote in his Weibo post.
Dr. Li, who had become less active on social media in recent days, liked an online poll on Weibo on Feb. 3 about whether people were back to work after vacation. According to a friend, the technology-obsessed Dr. Li’s online posts ranged from foldable Huawei mobile devices to hidden tricks with the iPhone, funny cat videos and pictures of his breakfast.
Even after he became infected, Dr. Li vowed to return to the front lines of the fight against the virus.
“The outbreak is still spreading,” he wrote on his verified account on Tencent News. “I don’t want to be a deserter.”
Updated: 2-17-2020
Did Coronavirus Originate In Chinese Government Laboratory?

Scientists Believe Killer Disease May Have Begun In Research Facility 300 Yards From Wuhan Wet Fish Market
-
- Beijing-Sponsored South China University Of Technology Concludes That ‘The Killer Coronavirus Probably Originated From A Laboratory In Wuhan’
It Points To Research On Bats And Respiratory Diseases Carried By The Animals At The Wuhan Center For Disease Control And The Wuhan Institute Of Virology
WCDC Is Just 300 Yards From The Seafood Market And Is Adjacent To The Hospital.
Chinese scientists believe the deadly coronavirus may have started life in a research facility just 300 yards from the Wuhan fish market.
A new bombshell paper from the Beijing-sponsored South China University of Technology says that the Wuhan Center for Disease Control (WHCDC) could have spawned the contagion in Hubei province.
‘The possible origins of 2019-nCoV coronavirus,’ penned by scholars Botao Xiao and Lei Xiao claims the WHCDC kept disease-ridden animals in laboratories, including 605 bats.
It also mentions that bats – which are linked to coronavirus – once attacked a researcher and ‘blood of bat was on his skin.’
The report says: ‘Genome sequences from patients were 96% or 89% identical to the Bat CoV ZC45 coronavirus originally found in Rhinolophus affinis (intermediate horseshoe bat).’
It describes how the only native bats are found around 600 miles away from the Wuhan seafood market and that the probability of bats flying from Yunnan and Zhejiang provinces was minimal.
In addition there is little to suggest the local populace eat the bats as evidenced by testimonies of 31 residents and 28 visitors.
Instead the authors point to research being carried out withing a few hundred yards at the WHCDC.
One of the researchers at the WHCDC described quarantining himself for two weeks after a bat’s blood got on his skin, according to the report. That same man also quarantined himself after a bat urinated on him.
And he also mentions discovering a live tick from a bat – parasites known for their ability to pass infections through a host animal’s blood.
‘The WHCDC was also adjacent to the Union Hospital (Figure 1, bottom) where the first group of doctors were infected during this epidemic.’ The report says.
‘It is plausible that the virus leaked around and some of them contaminated the initial patients in this epidemic, though solid proofs are needed in future study.’
And as well as the WHCDC, the report suggests that the Wuhan Institute of Virology could also have leaked the virus, as has previously been reported by MailOnline.
‘This laboratory reported that the Chinese horseshoe bats were natural reservoirs for the severe acute respiratory syndrome coronavirus (SARS-CoV) which caused the 2002-3 pandemic,’ the report says.
‘The principle investigator participated in a project which generated a chimeric virus using the SARS-CoV reverse genetics system, and reported the potential for human emergence 10. A direct speculation was that SARS-CoV or its derivative might leak from the laboratory.’
The report concludes that ‘the killer coronavirus probably originated from a laboratory in Wuhan.’
It comes as the outbreak has infected more than 69,000 people globally, with 1,665 deaths in China – most of these in the central province of Hubei.
Updated: 2-22-2020
Italy, South Korea Work to Contain Coronavirus Outbreaks
International health officials are worried about growing clusters of the illness outside of China.
South Korea reported a surge in coronavirus cases centered on a church where a thousand people have been exposed to the disease, as Italy worked to contain an outbreak in two clusters that by late Saturday had left about 50 people infected and two dead.
International health officials are worried about growing clusters of the illness outside of China, where the virus originated, including among people who have neither been to China nor been in contact with anyone who has.
Officials in both Italy and South Korea urged people to stay away from large gatherings. In Italy, officials ordered people in three small towns where the bigger cluster is centered to stay in their homes and canceled Masses, soccer matches and planned celebrations.
In South Korea, now the most virus-hit country outside of China, the number of confirmed coronavirus cases jumped to 433 by late Saturday afternoon, health officials said. The figure is more than double the count from just a day before, and almost 15 times higher than the tallies from Monday, according to the Seoul government. Officials have linked at least two deaths to the disease.
Health officials suspect that the surge is partly due to a large church service that an elderly woman who had contracted the disease attended in the southeastern city of Daegu, also home to a U.S. military garrison of 2,500 troops. More than 6,000 individuals are undergoing medical screening to test if they have been infected, South Korean officials said, suggesting the number of confirmed cases could go up in the coming days.
South Korean Prime Minister Chung Sye-kyun urged people in a nationally televised press conference to stop attending large gatherings, including religious services.
“I urge our citizens to temporarily refrain from going to events where many people are in close proximity to one another,” he said.
No American—military or civilian—has contracted the disease, said Army Col. Edward J. Ballanco this week.
Among the two dead in Italy, both of whom where over 70 years old, was a woman who caught the virus in an emergency room in one of the towns south of Milan, according to a health official. It was confirmed only after she died that she had the virus.
The outbreak near Milan appears to have taken off once a 38-year-old man who hadn’t traveled to China got sick, according to officials. He visited an emergency room several times with respiratory problems, but no special measures were taken and he was initially sent home with antibiotics because he wasn’t considered to be at risk.
Several people at the hospital, including some health workers, caught the virus, as did a number of people who frequented a bar where the 38-year-old had been. He is now at a hospital in critical, but stable condition.
Health officials said they are still looking for the person who passed the virus to the 38-year-old. They suspect a friend of his who returned to Italy from China in late January, but didn’t show symptoms or get sick, according to Italian press reports.
Before the two clusters that broke out late in the week, the only three cases in Italy were a Chinese couple on vacation and an Italian who had recently returned from Wuhan, the virus epicenter. The couple, after passing a period in critical condition, is recovering while the Italian is almost fully recovered.
The focal point of the outbreak is the town of Codogno, about 40 miles southeast of Milan. Television footage showed the streets of Codogno deserted on Saturday afternoon, when normally many people would be out.
On Friday, health officials expressed hope that the cluster would be contained in the immediate area of the three towns. That was short-lived, with Attilio Fontana, the president of Lombardy, the region where Milan is the capital, saying in a press conference that a case had emerged in a city 20 miles away.
An ordinance issued by the government in Rome ordered people who have had direct contact with infected people to self-quarantine in their homes for 14 days. The ordinance also called upon people returning from the part of China most-affected by the virus to alert local health authorities, who will then manage an eventual quarantine.
Italian Premier Giuseppe Conte said other extraordinary measures could be enacted. He didn’t give details.
There has been no word what officials will order for the area around Codogno on Monday. The town is in Italy’s industrial heartland, where numerous companies are already reeling because of an inability to get key parts from China.
The second cluster, where about 10 cases emerged in the past two days and one man died, is centered around a small town in the Veneto region near Venice.
Health officials have so far not found a link between the two clusters.
Updated: 2-24-2020
Coronavirus’s Global Spread May Not Be Contained, WHO Says
Number of new cases in China declines, but it isn’t clear whether outbreak can be stopped from spreading globally, World Health Organization says.
The World Health Organization said Monday it isn’t yet clear whether the new coronavirus, which has sparked large outbreaks in several countries, can be stopped from spreading further.
Yet the agency said the virus isn’t causing a pandemic so far, and the epidemic it sparked in China has peaked and the number of new cases there is declining. A pandemic indicates widespread transmission on multiple continents, according to the WHO.
“We are encouraged by the continued decline in [new] cases in China,” Tedros Adhanom Ghebreyesus, the WHO’s director-general, said at a news conference. “The key message that should give all countries hope, courage and confidence is that this virus can be contained,” he added.
A team of international experts, led by the WHO, who have been in China over the past week concluded that the epidemic there peaked between Jan. 23 and Feb. 2 and has been falling steadily since, Dr. Tedros said. For example, China reported 2,590 new cases on Feb. 2, and 460 new cases on Feb. 24.
The team found that “the measures taken in China have averted a significant number of cases,” he said. He didn’t specify which measures in particular.
Yet Dr. Tedros also called outbreaks that have arisen in recent days in Italy, Iran and South Korea “deeply concerning.”
There are now 79,436 confirmed cases in 28 countries of Covid-19, the disease caused by the coronavirus, including 2,641 deaths, he said.
Some infectious disease experts have said they believe it will now be impossible to stop the contagious respiratory virus from spreading more widely globally, given that it appears to spread easily and that people who are asymptomatic or not very ill can transmit it.
The WHO said it isn’t yet clear whether that is the case.
“There is still a possibility that we can contain the virus and interrupt its transmission,” Michael Ryan, chief of health emergencies for the WHO, said at the news conference. “But the virus may settle down into an endemic pattern of transmission, into a seasonal pattern or could accelerate into a full-blown global pandemic. At this point it is not possible to say which of those realities is going to happen.”
The WHO bases its assessment about whether a disease is causing a pandemic on its geographic spread, severity of the disease and its impact on society, Dr. Tedros said.
“For the moment we are not witnessing the uncontained global spread of this virus, and we are not witnessing large-scale severe disease or death,” he said.
Updated: 3-8-2020
The Coronavirus Hunter Is Racing for Answers in a Locked-Down Lab

A University of North Carolina scientist who has been chasing viruses for decades may hold the key to a cure.
The deadly coronavirus arrived by courier on February 6, delivered to a windowless air-locked laboratory in a secret location on the University of North Carolina at Chapel Hill campus. It came sealed in two 500-microliter vials, wrapped inside plastic pouches, placed inside a third sealed plastic container, all packed with dry ice.
A team of scientists — protected head-to-toe by Tyvek body suits with battery-powered respirators — opened the vials, got down to work and haven’t stopped since. Members of an elite lab of virologists at the university’s Gillings School of Global Public Health, their mission is to come up with a drug to treat the pathogen that has already infected over 90,000 people and killed more than 3,000.
For veteran researcher and lab leader Ralph Baric, it’s the moment he has both long feared and expected. As early as the 1990s, Baric’s work was raising red flags: Coronaviruses — so named for the crown-like spikes that help them invade cells — had an extraordinarily high ability to mutate and adapt. That suggested new coronaviruses might spread into humans in the future. In one study, he showed coronaviruses that infected mice could rapidly adapt to invade hamster cells.
“It was not that difficult to evolve strains that could jump between species,” Baric says.
Almost 30 years later, that’s exactly what’s occured with the deadly new coronavirus known as SARS-CoV-2. Scientists believe it began in a cave somewhere in China, with bats flying off to spread the virus to other animals in the wild. Some of those animals eventually wound up in one of China’s open-air, or so-called wet, markets where live animals are caged in close proximity — a perfect setting for transmitting viruses to humans.
Until two months ago, Baric was little known outside academic circles. When he began his career, coronaviruses were understood as causing little more than a common cold in people. But his work has suddenly taken on new urgency with the appearance of the new coronavirus.
Baric’s 30-person team was one of the first in the U.S. to receive samples of the virus isolated from a patient in Washington by the Centers for Disease Control and Prevention. A handful of other labs are also racing to find anything that might slow the virus’ spread or ease its symptoms, the University of Maryland School of Medicine and Vanderbilt University School of Medicine among them.
Baric’s team is growing as much of the virus as it can to test possible drugs for their ability to inhibit it inside human lung cells in a test tube. This first round of testing will likely wrap up soon. If it works, scientists will test a slew of new drugs in mice that have been engineered to carry human lung receptors that the coronavirus can infect.
“Now that we have the virus, it is a lot of people working all the time,” says Lisa Gralinski, an assistant professor under Baric.
The pace is just as frenzied at the few other labs with samples. “It has been 18-to-20-hour days for the last two months,” says Matthew Frieman, a University of Maryland virologist and a Baric protégé, who was also among the first to receive the virus.
Researchers at the World Health Organization have called Gilead Sciences Inc.’s remdesivir, developed with Baric’s assistance, as the most promising agent identified so far against the new virus. Trials of the drug are underway in hard hit areas of China, and Gilead says it expects results by April.
To speed the efforts, government agencies are redirecting existing funds to bolster coronavirus research. Congress agreed on March 4 to spend $7.8 billion in emergency funding, some of which will be for drug and vaccine development. The government is working with Regeneron Pharmaceuticals Inc. and Johnson & Johnson to create new drugs or identify existing ones in the hope of quickly finding something that can slow down the global scourge.
“There are hundreds and hundreds of new technologies. Our job is to comb through those as quickly as possible,’’ says Rick Bright, director of the U.S. Biomedical Advanced Research and Development Authority, a branch of the Department of Health and Human Services. “The ultimate goal is to get as many ideas going” as they can.
No one is more aware of that urgency than Baric, who stands out as a leader in the campaign. Suddenly at the center of the action, he seems uneasy in the limelight, preferring to focus on his work in his office piled high with research papers, virology books and framed patents he hasn’t gotten around to hanging up.
His warnings about the dangers of coronaviruses were first proven on the mark when SARS, or Severe Acute Respiratory Syndrome, swept through China and other countries in 2002 and 2003, eventually killing almost 800. The virus originated in bats and is thought to have passed through palm civets on its way to people.
By 2012, a deadly pathogen from camels, Middle East respiratory syndrome (MERS), began killing people in the region. Eventually, more than 850 died.
In 2015, Baric and his colleagues were able to show that SARS-like viruses in Chinese horseshoe bats posed a particular threat to cause a new outbreak. The virus spike in the bat coronavirus was unusually adaptable, allowing it to recognize receptors in multiple species, including human lung cells.
Over the last five years, Baric, working closely with Vanderbilt University infectious-disease specialist Mark Denison, tested almost 200,000 drugs against SARS, MERS and other bat coronavirus strains. He found at least two dozen that appeared to hinder the virus.
Among the most promising was Gilead’s remdesivir, a drug that fared poorly when used against a recent Ebola outbreak in Africa. In the lab, it worked against numerous coronavirus strains, including SARS and other bat coronaviruses that are similar to the new strain. Every coronavirus it was tested on, “it had high potency and efficacy,” Denison says.
That work was fortuitous. In early January, Baric got an urgent call from an infectious-disease colleague to send his unpublished data on remdesivir to colleagues in China who were dealing with a then-mysterious outbreak. Baric says he “was shocked” to see how fast the coronavirus was spreading.
Since then, work at his lab has been virtually nonstop. Each scientist puts in from one to six hours inside two different clean rooms equipped to handle the virus. The lab’s workday begins at 6 a.m. and often goes until 11 p.m. Individual sessions are short for safety and practical reasons — researchers aren’t permitted to eat, drink or visit the bathroom once inside the lab. Everyone has to pass an FBI background check and undergo months of safety training.
Scrubbing up and gowning takes 15 minutes, a laborious process that includes putting on multiple layers of Tyvek suits, nitrile gloves and booties, along with an air-purifying respirator powered by a battery that belts around the waist. Exiting the lab is just as exacting and involves researchers spraying themselves down repeatedly with 70% alcohol as they take off each layer of protective clothing to kill any stray viral particles.
The workload, Baric says, is “overwhelming” as companies and researchers around the globe turn to his lab for help. He’s narrowed down the search to about 100 drugs that are likely to show promise against coronaviruses. Even if the Gilead drug works — a big if — it would have drawbacks: It can’t be offered in pill form, for instance, but must be infused in a hospital or doctor’s office.
More crucially, other drugs may need to supplant it to fight even newer coronaviruses. So Baric is moving forward to find yet other treatments that could succeed against the numerous coronaviruses now lurking in bats and other mammals, poised to jump to humans at any moment.
“The goal of our program is to find broad-based inhibitors that work against everything in the virus family,” Baric says . That makes the challenge sound matter of fact, but Baric knows the road ahead will be long and hard. “I have a lot people who are really tired,” he says. “They are working really hard.”
Updated: 3-10-2020
Fever-Detecting Goggles and Disinfectant Drones: Countries Turn to Tech to Fight Coronavirus
But with high-tech solutions come bugs, glitches and human error.

Drones spray disinfectant over South Korea. Police wear thermal imaging goggles to detect fevers in China. And a chatbot fields coronavirus questions in Australia.
The tech industry has long touted how ubiquitous connectivity, flashy gadgets and big data can improve people’s lives. The novel coronavirus epidemic is putting that bold promise to the test.
Health officials across Asia-Pacific, home to the first waves of virus contagion, have sought to repurpose existing technology to combat the fast-spreading virus. They are using smartphone-location tracking to piece together movements of suspected cases, developing government-run apps to monitor individuals’ health and keeping an eye on people’s temperature in the street with thermal goggles.
These new responses supplement traditional tactics such as quarantining sick people and canceling mass public events. But the tech-savvy tactics have yet to demonstrate broadly whether they are more game-changer than gimmick. Still, countries elsewhere might look to these solutions as the epidemic spreads.
The global number of confirmed coronavirus cases rose above 110,000 on Monday, according to data compiled by Johns Hopkins University, with infections found in 108 countries and regions.
In South Korea, the country hit hardest by the virus after China and Italy, the government rolled out a “Self-Quarantine Safety Protection” tracking app to keep digital eyeballs on the roughly 30,000 people officials told to stay home for two weeks. If a person brings their phone out of the permitted area, a mobile alert gets beamed to the individual and their government case officer.
The technology comes with a rub: It isn’t available on Apple Inc.’s iPhones until March 20. The app only works on handsets that run Google’s Android operating system used by hometown brands, Samsung Electronics Co. and LG Electronics Inc. Voluntary downloads since Saturday’s launch have been low, a government spokesman said.
In Singapore, a Southeast Asian country hit in the early stages of the virus outbreak, health officials are asking citizens to monitor their own movements with the QR code, the black-and-white bar code used for mobile payments.
A scan of these codes, found in taxis, office lobbies, tourist attractions and colleges, bring people to a webpage where they are asked to input their names, contact details and on occasion declare their health status. The voluntary scans allow authorities to reverse engineer a citizens’ whereabouts in case they fall ill or come into contact with a patient.
In Singapore’s Nanyang Technological University, where students, staff and visitors scan such bar codes to leave a digital trail of the locations they visit in the university, the data helped the university probe whether any of their 33,000 students had come into contact with a cleaning contractor who worked in the school after that person was diagnosed with Covid-19, said Tan Aik Na, a senior vice president at the university.
The system has limits. Claudia Thong, a 21-year-old Singapore university student, scans QR codes pasted on the front doors and interiors of classrooms each time she attends lessons. Some students, however, can’t be bothered to scan the codes, she said. Faculty and staff have been asked to remind their students and guests to perform the QR code check-in, the university said in a statement.
Australia’s health department directs worried citizens to a virtual assistant named “Sam.” But inquiries for “coronavirus” go unrecognized, with the site suggesting the correct spelling is the two-word, “corona virus.” A follow-up question about anxieties relating to “corona virus” produced suggestions that had nothing to do with the respiratory illness.
The chatbot will soon be updated to refer people to Covid-19 resources, an Australian health-department spokesman said.
In China, where the largest Covid-19 outbreak has occurred, cities have deployed a variety of eye-catching technologies to diagnose and contain illness. Through measures such as social distancing and isolation, China has managed to limit the outbreak mostly to Hubei province, where the infectious disease emerged and where the majority of cases have occurred.
Unmanned aerial vehicles, typically used to spot forest fires or for police surveillance, can now scan crowds in China and spot someone hundreds of feet away running a fever, said Kellen Tse, deputy general manager for Shenzhen Smart Drone UAV Co., a drone company working with two Chinese provinces. The drone, which uses thermal imaging, sends alerts about those unwell to on-the-ground officials.
“China is unlike other countries,’ Mr. Tse said. “We have a large population, that’s why we’ve turned to technology to be more efficient.”
In Shanghai, digital devices are attached to the doors of those sequestered, according to the city’s state-television channel. People are allowed to go out to empty their trash and pick up deliveries, but unauthorized door movements trigger an alarm to the neighborhood police station, a policewoman told the broadcaster in an interview.
Chinese technology firm Baidu Inc. said this month that it helped develop an algorithm for Beijing subway officials to single out commuters not wearing masks. The image-recognition algorithm, which Baidu developed and tested seven days after a request from the city’s metro administration, runs on the video feeds from subway cameras and flags individuals without a mask or who don’t wear one properly.
Shenzhen, China’s tech-manufacturing center, requests that drivers entering the city scan a QR code and leave their contact details and travel history. Police officers wear thermal helmets and goggles to identify pedestrians who may be unwell, the Shenzhen government said on social media.

But the new-age tactics have their limitations. Commercial drones can only fly for about 20 minutes before needing a lengthy recharge, and the tech-heavy defenses are expensive, said Peter Fuhrman, a Shenzhen resident and chairman of China First Capital, a boutique investment bank. He credits the conventional response of the masses of volunteers and paid monitors deployed in Chinese neighborhoods with thwarting the virus.
“Fittingly, people, not machines, made all the difference here,” said Mr. Fuhrman, who has stayed in Shenzhen since the country’s outbreak began in January.
In South Korea’s hard-hit city of Daegu, private drone companies have been deployed to help disinfect public places at the local government’s request. A single drone can load around 2.5 gallons of disinfectant and spray an area of up to 105,000 square feet—or about the size of a typical Walmart store.
“It takes about 10 to 12 minutes to use it all up,” a Daegu city official said.
Updated: 3-22-2020
These Drugs Are Helping Our Coronavirus Patients
The evidence is preliminary on repurposing two treatments. But we don’t have the luxury of time.
A flash of potential good news from the front lines of the coronavirus pandemic: A treatment is showing promise. Doctors in France, South Korea and the U.S. are using an antimalarial drug known as hydroxychloroquine with success. We are physicians treating patients with Covid-19, and the therapy appears to be making a difference. It isn’t a silver bullet, but if deployed quickly and strategically the drug could potentially help bend the pandemic’s “hockey stick” curve.
Hydroxychloroquine is a common generic drug used to treat lupus, arthritis and malaria. The medication, whose brand name is Plaquenil, is relatively safe, with the main side effect being stomach irritation, though it can cause echocardiogram and vision changes. In 2005, a Centers for Disease Control and Prevention study showed that chloroquine, an analogue, could block a virus from penetrating a cell if administered before exposure. If tissue had already been infected, the drug inhibited the virus.
On March 9 a team of researchers in China published results showing hydroxychloroquine was effective against the 2019 coronavirus in a test tube. The authors suggested a five-day, 12-pill treatment for Covid-19: two 200-milligram tablets twice a day on the first day followed by one tablet twice a day for four more days.
A more recent French study used the drug in combination with azithromycin. Most Americans know azithromycin as the brand name Zithromax Z-Pak, prescribed for upper respiratory infections. The Z-Pak alone doesn’t appear to help fight Covid-19, and the findings of combination treatment are preliminary.
But researchers in France treated a small number of patients with both hydroxychloroquine and a Z-Pak, and 100% of them were cured by day six of treatment. Compare that with 57.1% of patients treated with hydroxychloroquine alone, and 12.5% of patients who received neither.
What’s more, most patients cleared the virus in three to six days rather than the 20 days observed in China. That reduces the time a patient can spread the virus to others. One lesson that should inform the U.S. approach: Use this treatment cocktail early, and don’t wait until a patient is on a ventilator in the intensive-care unit.
A couple of careful studies of hydroxychloroquine are in progress, but the results may take weeks or longer. Infectious-disease experts are already using hydroxychloroquine clinically with some success. With our colleague Dr. Joe Brewer in Kansas City, Mo., we are using hydroxychloroquine in two ways: to treat patients and as prophylaxis to protect health-care workers from infection.
We had been using the protocol outlined in the research from China, but we’ve switched to the combination prescribed in the French study. Our patients appear to be showing fewer symptoms.
Our experience suggests that hydroxychloroquine, with or without a Z-Pak, should be a first-line treatment. Unfortunately, there is already a shortage of hydroxychloroquine. The federal government should immediately contract with generic manufacturers to ramp up production. Any stockpiles should be released.
As a matter of clinical practice, hydroxychloroquine should be given early to patients who test positive, and perhaps if Covid-19 is presumed—in the case of ill household contacts, for instance. It may be especially useful to treat mild cases and young patients, which would significantly decrease viral transmission and, as they say, “flatten the curve.”
Emergency rooms run the risk of one patient exposing a dozen nurses and doctors. Instead of exposed health workers getting placed on 14-day quarantine, they could receive hydroxychloroquine for five days, then test for the virus. That would allow health-care workers to return to work sooner if they test negative.
President Trump touted hydroxychloroquine in his Thursday press conference as a potential treatment, which is a welcome move. And this isn’t only about treatment. Rapid and strategic use of these drugs could help arrest the spread of the disease.
We have decades of experience in treating infectious diseases and dealing with epidemics, and we believe in safety and efficacy. We don’t want to peddle false hope; we have seen promising drugs turn out to be duds.
But the public expects an answer, and we don’t have the luxury of time. We have a drug with an excellent safety profile but limited clinical outcomes—and no better alternatives until long after this disaster peaks. We can use this treatment to help save lives and prevent others from becoming infected. Or we can wait several weeks and risk discovering we didn’t do everything we could to end this pandemic as quickly as possible.
Updated: 3-31-2020
8 Experimental Coronavirus Treatments to Watch
Identifying a treatment for the novel coronavirus is perhaps the most vital scientific problem of the moment. While vaccines could bring an end to the pandemic, none is likely to be widely available for a year or more. Therapeutics offer the possibility of a shorter-term answer; one that could reduce the frightening death toll.
It is unlikely that scientists will find a silver bullet cure to the novel coronavirus. So drug companies and other labs are working on a range of approaches to give doctors a variety of tools. Some of the drugs seek to stop the virus itself; others only aim to treat the potentially deadly complications it can cause. The Milken Institute, which is tracking the various efforts, counts 75 different possible treatments or efforts to discover a treatment. Here are some of the most important.
Remdesivir
Status: In human trials, with some data expected in April.
Remdesivir, a drug designed by Gilead (ticker: GILD) to treat Ebola, is a type of antiviral called a nucleotide analog. The drug has drawn substantial interest as a possible Covid-19 treatment, so much so that the company had to stop accepting requests for emergency access to it from Covid-19 patients. Remdesivir is currently the subject of at least seven clinical trials in Covid-19 patients, according to the Milken Institute. Clinical data from two Phase 3 trials of the drug are expected in April. If it is proven safe and effective, challenges remain: Remdesivir is delivered intravenously, which means it will likely only be used for severe cases of the disease.
Chloroquine and Hydroxychloroquine
Status: In human trials. The FDA has issued an Emergency Use Authorization to allow it to be used in certain Covid-19 patients.
These closely-related antimalaria drugs have emerged as the most controversial potential Covid-19 treatments. Data supporting the efficacy of the drugs remains thin. As recently as last Tuesday, an FDA official cautioned that the agency needs to “ask, in a judicious way, what are the data that support this particular intervention.” But interest in the drugs has been tremendous, in part due to President Donald Trump’s touting of hydroxychloroquine’s potential in speeches and on Twitter. The FDA said March 30 that it had put out an Emergency Use Authorization allowing hydroxychloroquine sulfate and chloroquine phosphate from the Strategic National Stockpile to be used in teen and adult patients hospitalized with Covid-19. Meanwhile, the drugs are the subject of dozens of clinical trials, often in combination with other medications.
Kevzara
Status: In human trials.
Kevzara, an arthritis drug sold by Sanofi (SNY) and Regeneron (REGN), is being tested in two separate Phase 2/3 trials in hospitalized patients with severe Covid-19. The drug, a so-called IL-6 inhibitor, may be able to tamp down inflammation in the lungs that, in serious Covid-19 cases, can lead to death.
Actemra
Status: In human trials. Chinese health officials recommend its use in some Covid-19 patients.
Actemra is a Roche (RHBBY) arthritis drug that, like Kevzara, is an IL-6 inhibitor. Like Kevzara, it is being studied for its potential to manage Covid-19 side effects in patients who develop pneumonia. China’s National Health Commission includes Actemra in its recommended Covid-19 treatment plan for patients with pneumonia.
Kaletra
Status: In human trials.
Kaletra, an HIV drug sold by AbbVie (ABBV), returned disappointing results in a Covid-19 trial in mid-March, but more trials are ongoing. The drug is a combination of two antivirals, called lopinavir and ritonavir, and first received FDA approval as an HIV treatment in 2000. AbbVie has decided not to enforce its patent on the drug, according to the Financial Times.
Regeneron antibody program
Status: Could start human trials by early this summer.
When the immune system conquers a virus, it creates proteins called antibodies that can identify and neutralize that virus if it turns up again. Various companies are working on identifying antibodies that can neutralize the virus that causes Covid-19, and turning those antibodies into a drug. Regeneron has identified hundreds of relevant antibodies from genetically modified mice, and from humans who have recovered from Covid-19. The company used the same technique to develop an Ebola drug. It says it will pick two of the antibodies to test as a cocktail treatment. It plans to begin manufacturing in April, and to start clinical trials by early in the summer.
Vir Biotechnology antibody program
Status: Human trials expected to begin in three to five months.
Vir Biotechnology (VIR) is working on a Covid-19 antibody therapy with Biogen (BIIB) and the Chinese firm WuXi Biologics. The company says the antibody it has identified can neutralize the virus that causes Covid-19 in tests in the lab. Biogen has signed on to help develop and manufacture the antibody, as has WuXi. Vir said on March 25 it expected human trials to begin within three to five months.
Vir Biotech siRNA program
Status: Preclinical.
Vir is also working with the biotech firm Alnylam Pharmaceuticals (ALNY) to develop Covid-10 drugs that use a technique known as RNA interference to go after the virus. The drug would use molecules called small interfering RNA that could potentially stop messenger RNA molecules from carrying instructions to make disease-causing proteins. Alnylam has synthesized hundreds of siRNA molecules targeting the genomes of the virus that causes Covid-19; Vir will now evaluate those molecules in the lab to select one to test.
Updated: 4-5-2020
Two Promising Approaches
Given the enormous public-health and economic stakes, it is worth doing whatever it takes to move such a drug to market. There are two promising approaches, and both could be available soon if government and private industry do things right. It’s time to place some firm bets and put resources behind these experimental treatments.
One approach involves antiviral drugs that target the virus and block its replication. Think of medicines for treating influenza, HIV or cold sores. The drugs work by blocking the mechanisms that viruses use to replicate. Dozens of promising antiviral drugs are in various stages of development and could be advanced quickly. The one furthest along is remdesivir, from Gilead Sciences. There’s evidence from clinical experience with Covid-19 patients that it could be effective.
The other approach involves antibody drugs, which mimic the function of immune cells. Antibody drugs can be used to fight an infection and to reduce the risk of contracting Covid-19. These medicines may be the best chance for a meaningful near-term success.
Antibody drugs are based on the same scientific principles that make “convalescent plasma” one interim tactic for treating the sickest Covid-19 patients. Doctors are taking blood plasma from patients who have recovered from Covid-19 and infusing it into those who are critically ill. The plasma is laden with antibodies, and the approach shows some promise. The constraint: There isn’t enough plasma from recovered patients to go around.
Antibody drugs are engineered to do the same thing as convalescent plasma, but because they’re synthesized, they don’t depend on a supply of antibodies from healed patients. Biotech companies would manufacture them in large quantities using recombinant technology, the same approach behind highly effective drugs that target and prevent Ebola, respiratory syncytial virus and other infections. The antibodies can also be a prophylaxis given to those exposed to Covid-19, or to prevent infection in vulnerable patients, such as those on chemotherapy. These drugs could protect the public until a vaccine is available.
Updated: 4-14-2020
GlaxoSmithKline, Sanofi Team Up for Coronavirus Vaccine
Drug giants hope combining efforts will allow them to pump out hundreds of millions of doses by next year.
Sanofi SA and GlaxoSmithKline PLC are joining forces to develop a coronavirus vaccine in a collaboration that—if successful—could pump out hundreds of millions of doses by the second half of 2021.
The partnership—a first between two major pharmaceutical companies to fight the pandemic—brings together existing efforts at the drug giants. It will combine Sanofi’s work reviving a shelved SARS vaccine with Glaxo’s expertise in developing “adjuvants,” or ingredients that boost the immune response to a vaccine.
The collaboration seeks to bypass a big hurdle facing the dozens of efforts under way at academic laboratories and startups: a lack of manufacturing capacity that could severely limit the number of people who could receive any vaccine.
The companies say their combined manufacturing capacity, plus the potential for an adjuvant to lower the required vaccine dose per patient, means they could quickly ramp up production if their candidate is successful. Glaxo, the biggest vaccine maker in the world by revenue, and Sanofi, the fourth largest, say they could produce hundreds of millions of doses of vaccine by the second half of next year.
“The advantages are we both have scale in manufacturing,” said Glaxo Chief Executive Emma Walmsley. “These are both proven pandemic technologies whether their antigen [the protein that forms the basis of Sanofi’s vaccine] or our adjuvant.”
Ms. Walmlsey said Glaxo’s pandemic adjuvant was able to quadruple the number of doses of vaccine it could manufacture for the H1N1 flu pandemic in 2009. Meanwhile, Sanofi’s vaccine is based on technology already used in its approved flu vaccine.
Still, the challenges of developing a successful vaccine mean Glaxo and Sanofi—like all vaccine developers—face steep odds. Typically it takes around a decade to develop a new vaccine with fewer than one in 10 candidates proving themselves in clinical trials. So far, there are no approved vaccines or treatments for Covid-19.
“Starting with proven pandemic technologies definitely improves the odds,” said Roger Connor, who leads Glaxo’s vaccines business. “The world wants to see confirmation that the challenge is being solved, but as with all research there’s risk involved with this.”
The companies didn’t disclose the commercial terms of their partnership.
Glaxo and Sanofi lag some of the smaller players in the fight against coronavirus. Moderna Inc. is already testing its vaccine in humans and a vaccine under way at the University of Oxford is to start human trials within weeks. Glaxo and Sanofi say their vaccine should reach that stage by the second half of 2020.
Glaxo and Sanofi are also working with smaller companies to fight the virus. Glaxo is providing adjuvants to several groups, including Clover Biopharmaceuticals, a Chinese biotech developing a vaccine. Sanofi is working with Translate Bio, a U.S.-based biotech, on another potential vaccine.
The rapid spread of Covid-19 around the world means that even if Glaxo and Sanofi can develop and scale up a product quickly, they are unlikely to be able to meet global vaccine demand.
“The world is going to need multiple vaccines to deal with the scale, and some are going to fail through the development process,” said Mr. Connor. “Multiple shots on goal is exactly the right approach and across technology as well.”
Updated: 7-22-2020
Pfizer, BioNTech Get $1.95 Billion Covid-19 Vaccine Order From U.S. Government
Vaccines to be provided to Americans free of charge; companies didn’t specify pricing details.
The U.S. has agreed to pay Pfizer Inc. and BioNTech nearly $2 billion to secure 100 million doses of their experimental Covid-19 vaccine to provide to Americans free of charge.
Under the $1.95 billion agreement, the U.S. Department of Health and Human Services and the Defense Department will receive 100 million doses of the vaccine should it be cleared by regulators, and can also acquire an additional 500 million doses. The vaccine, which has shown promising preliminary results in small groups of patients, is set to enter late-stage testing this month.
No Covid-19 vaccine in development has proven to work safely yet. The U.S. and other governments are spending billions of dollars to secure potential Covid-19 vaccines and treatments should they prove safe and effective. The race has countries scrambling as they try to secure enough vaccines and the supplies to transport them.
As part of its Operation Warp Speed program, the U.S. has already struck agreements with other vaccine developers to secure doses, including a $1.2 billion deal with AstraZeneca PLC for at least 300 million doses of a vaccine developed by University of Oxford researchers. A $1.6 billion agreement with Novavax Inc. will fund clinical studies of its experimental vaccine and establish large-scale manufacturing of doses.
The agreement with Pfizer and BioNTech is the largest from the U.S. so far to secure Covid-19 vaccine supplies. It won’t fund any research and development, according to the companies, unlike other deals that helped pay for testing and the scale-up of manufacturing capacity.
While the vaccines may be provided to Americans free of charge, New York-based Pfizer and Germany’s BioNTech didn’t specify pricing details. Analysts estimated the vaccine’s price at $19.50 per dose, based on the announcement. The price is in line with what the private sector pays for flu shots.
Pfizer has previously said it expects to make a profit from its Covid-19 vaccine.
The Pfizer and BioNTech vaccine is set to enter a late-stage, 30,000-person study this month. If the testing is successful and the vaccine is proven to work safely, Pfizer and BioNTech said they expect to seek emergency use authorization or some form of regulatory approval as early as October.
Earlier this week, the two companies released additional positive early-stage results of a trial in Germany, which supported results from a corresponding U.S. trial. The companies are studying at least four vaccine candidates.
The companies expect to manufacture globally up to 100 million doses by the end of 2020 and potentially more than 1.3 billion doses by the end of 2021, subject to final dose selection from their clinical trial.
The Pfizer-BioNTech vaccine uses an innovative gene-based technology known as messenger RNA. Messenger RNA, or mRNA, carries instructions from DNA to the body’s cells to make certain proteins. An mRNA vaccine has never been approved to prevent any infectious disease.
Aside from AstraZeneca and Novavax, other companies getting U.S. funding for coronavirus drug and vaccine programs are Moderna Inc., Johnson & Johnson and Regeneron Pharmaceuticals Inc.
Updated: 7-27-2020
Moderna, Pfizer Coronavirus Vaccines Begin Final-Stage Testing
Drugmakers to enroll 30,000 subjects across U.S., in separate trials to determine whether their vaccines prevent symptomatic Covid-19.
Two of the most advanced experimental coronavirus vaccines entered the pivotal phase of their studies on Monday, with the first subjects receiving doses of vaccines developed by Moderna Inc. and Pfizer Inc.
Researchers evaluating the vaccines plan to enroll 30,000 people in separate last-stage, or phase 3 trials, results of which will determine whether the vaccines protect against symptomatic Covid-19, and whether they should be cleared for widespread use.
Moderna’s two-dose vaccine will be administered at sites across the U.S. The Cambridge, Mass., company also received an additional commitment of up to $472 million from the federal government to support the large study, on top of the $483 million in funding earlier in the year for development, testing and preparations to manufacture at large scale.
Moderna, which codesigned the vaccine with the National Institute of Allergy and Infectious Diseases, previously reported promising results of the first study of the vaccine, showing it induced immune responses and was generally safe in a small number of people.
The new phase 3 trial, titled Cove, is being conducted at nearly 90 sites in the U.S., including many in states such as Texas where the virus has surged in recent weeks.
Pfizer’s trial, which will begin in the U.S. but expand overseas to include about 120 sites, will evaluate a vaccine developed with partner BioNTech SE. The shot is one of four candidates the companies evaluated.
As vaccines proceed through the final stages of testing, countries are jockeying to secure enough shots, should they prove safe and effective, and the supplies to transport them.
Last week, the U.S. agreed to pay Pfizer and BioNTech nearly $2 billion to secure 100 million doses of their experimental Covid-19 vaccine to provide to U.S. patients free of charge. Pfizer is targeting October to file for regulatory approval or an emergency use authorization.
No vaccine has proven to work safely against the coronavirus. Many vaccines developed to target other pathogens have failed in testing, including in phase 3 trials.
U.S. officials said Monday it was the fastest time from the design of a new vaccine to the start of phase 3 testing in the U.S. NIAID researchers helped design the vaccine, code-named mRNA-1273, in January after the genetic code of the virus was posted to a public database.
“Yes, we’re going fast, but no, we are not going to compromise safety or efficacy,” National Institutes of Health Director Francis Collins said on a conference call with reporters Monday. NIAID is part of the NIH.
The start of the pivotal Moderna vaccine trial is the latest sign that the most advanced coronavirus vaccine candidates are moving into the final stages of testing, and could be ready for wider use before year’s end if results are positive.
A vaccine co-developed by the University of Oxford and AstraZeneca PLC started a large study in May in the U.K., and is due to enter a phase 3 study in the U.S. in August.
Another company expected to be a major player in the vaccine hunt, Johnson & Johnson, started dosing participants in the first U.S. human study of its experimental shot Monday. A larger, phase 3 study of J&J’s vaccine may start in September.
The first four patients in Pfizer’s trial were injected Monday afternoon, a spokeswoman said.
In Pfizer’s study, half the participants will get a two-dose regimen of the vaccine three weeks apart, with the rest getting placebo shots. A week after the second shot, researchers will begin checking whether the vaccinated group develops symptoms more or less frequently than those who weren’t vaccinated.
Pfizer is testing a different vaccine than the one for which results were announced earlier this month. The one moving forward is more tolerable, with lower frequency of mild reactions such as fever and chills, while still generating an immune response, said William Gruber, senior vice president of Pfizer’s vaccine clinical research. Pfizer researchers believe this vaccine would offer better protection, particularly for older adults.
In addition to enrollment in Covid-19 hot spots, Pfizer plans to enroll people who are at high risk, including those with underlying health conditions, older adults and racial or ethnic minorities, Dr. Gruber said. He said such enrollment plans could elicit answers about efficacy quickly.
Moderna hopes its large study, which is being conducted in collaboration with the NIH, will yield positive results and possible vaccine availability in the fall, with J&J aiming for similar milestones by early 2021.
In the new Moderna vaccine study, about half of the adult volunteers will receive two shots of the vaccine four weeks apart, while the other half will get two doses of a placebo. Starting two weeks after the second dose, researchers will track whether the rate of symptomatic Covid-19 disease in the vaccinated group is lower than in unvaccinated people.
NIAID Director Anthony Fauci said researchers may learn in November or December whether the Moderna vaccine works, but the answer could come sooner, depending on infection rates in the places where the study is being conducted.
Moderna has been manufacturing doses even before getting an answer on efficacy, but if it gets a green light from the government for wider user, it is unlikely there will be enough doses for all Americans right away.
Chief Executive Stéphane Bancel said there could be enough doses initially for “millions and millions” of people, which could be prioritized for at-risk groups like health-care workers.
The NIH and the Centers for Disease Control and Prevention have asked a National Academy of Medicine committee to come up with a plan for equitable allocation of any effective Covid-19 vaccines.
Moderna expects a wider rollout of the vaccine next year if the study is positive. It says it is on track to deliver about 500 million doses a year, and possibly up to one billion doses, starting in 2021.
Moderna shares rose 9% to $79.91 in trading Monday.
Updated: 7-30-2020
Drugmakers Race To Build Covid-19 Vaccine Supply Chains
Supply shortages, specialized handling and tight transportation capacity will make it harder to distribute hundreds of millions of vaccine doses.
Pharmaceutical companies that are racing to develop vaccines for the coronavirus are already working behind the scenes to build the supply chains needed to deliver their drugs to billions of people as rapidly as possible.
To serve global demand once a vaccine is approved, a complicated and high-stakes supply chain would kick into gear on a scale that the drug industry has rarely seen. The preparations involve lining up raw materials and factory capacity to manufacture a vaccine in large volumes, and the equipment needed to transport many millions of doses at once through distribution channels that will be subject to tight security and temperature controls.
The magnitude and speed of the effort creates the potential for lapses at each step that could cost invaluable doses.
Vaccines likely would be sent to hospitals, pharmacies, and central vaccination points, in the same way that medical teams have set up in parking lots, schools and other sites to provide testing for the virus that has, by Johns Hopkins University’s latest count, infected over 16 million people world-wide and killed over 661,000.
“We’ve never had to do something at this scale before,” said Remo Colarusso, vice president of supply chain at Janssen Pharmaceutical Companies, a company owned by Johnson & Johnson that says it is on the verge of clinical trials for a potential vaccine.
U.S. government is getting involved, allocating $10 billion for Operation Warp Speed, an initiative that aims to speed up vaccine development with the objective of distributing 300 million doses of coronavirus vaccine by January 2021. By comparison, drugmakers supplied 174.5 million doses of the flu vaccine between last September and February in the U.S., according to the Centers for Disease Control and Prevention.
Lawmakers are considering plans to bolster the vaccine effort with an additional $25 billion. “Once a vaccine has been successfully developed, how do you get all the production you need, and how do you get it out? That is a role we obviously will be playing a part in,” Senate Majority Leader Mitch McConnell (R., Ky.) said in July ahead of negotiations in Congress for the next round of coronavirus aid.
The planning comes as developers in several countries are reporting progress.
Three vaccine initiatives—University of Oxford researchers and AstraZeneca PLC; Pfizer Inc. and its German partner BioNTech SE ; and China’s CanSino Biologics—all said last week their shots generated immune responses and appeared generally safe to use. Earlier this week, there were 25 potential vaccines in clinical evaluation and 139 in preclinical evaluation, according to the WHO.
Shoring Up Manufacturing, Distribution Channels
Some of the companies involved are building this supply chain for the first time.
Moderna Inc., the 10-year-old Cambridge, Mass.-based company earlier this week said it had started final-stage testing of a vaccine, had never sold a product on the market. Neither has Novavax Inc., a Gaithersburg, Md.-based drug developer that was awarded the biggest federal grant for vaccine manufacturing to date.
“Just because Novavax has yet to bring a product to market, doesn’t mean that we don’t have a team of people that does have experience and ability to do that,” said John Trizzino, executive vice president, chief business officer and chief financial officer at Novavax, which hired a manufacturing chief in June. Moderna didn’t respond to requests for comment.
Experienced drugmakers say they are moving to shore up their existing processes for making and shipping pharmaceuticals.
“Just every day, [we are] trying to do things that we normally do in a year, do them in months. Things that normally take months, do them in days,” said Pamela Siwik, vice president of a division in global supply at Pfizer Inc., which is developing vaccine candidates with BioNTech.
Pharmaceutical companies at the start will need to produce enough of what is known as the drug substance, the primary vaccine ingredient.
J&J is developing a vaccine that uses an inactivated cold virus to deliver a part of the drug. To manufacture it, the company plans to use the same type of bioreactor that it used for making an Ebola vaccine, which this month won critical regulatory approval in Europe, but at 90 times the scale, Mr. Colarusso said.
Other companies, including Moderna and Pfizer, are developing a novel type of vaccine that delivers mRNA, a type of genetic material. Making this drug substance in bulk would require smaller equipment than other methods, Ms. Siwik said. But formulating the drug substance requires a unique process, so Pfizer is designing new machinery with its vendors and modifying its plants to install equipment needed for the work, she added.
J&J has struck deals with U.S. contract drug manufacturers Emergent BioSolutions Inc. and Catalent Inc. and plans to expand manufacturing in Europe and Asia. The company will make a drug at sites around the world simultaneously for the first time, Mr. Colarusso said.
Once they have produced the final liquid vaccine, the pharmaceutical companies will need to fill vials with it, adding another hurdle to distribution.
Medical glass has been in short supply since before the pandemic, when China began to require containers for long-term storage of pharmaceutical products, and that shortage has worsened. In June, the U.S. awarded glass products manufacturer Corning Inc. $204 million to expand manufacturing capacity and produce vials for coronavirus vaccines.
J&J alone has bought 250 million vials, WSJ has reported previously. Pfizer went to its suppliers early to secure the containers, Ms. Siwik said.
After drugmakers fill the vials, they will turn to logistics providers experienced in handling pharmaceuticals to ship them to distributors or directly to medical providers.
Shipping Capacity In Question
Logistics operators could be another speed bump. They have struggled at times during the pandemic amid upheaval in demand—particularly for consumer products and medical gear—that has left companies scrambling to find warehousing and transportation space. Airfreight capacity, which will be crucial for moving a vaccine in the early days of distribution, has been hit particularly hard because thousands of passenger flights—which carry goods as well—have been grounded since the pandemic began.
“The logistics industry doesn’t have enough of anything—air capacity, ground handling personnel, specialized equipment—to handle this,” said Neel Jones Shah, executive vice president and global head of airfreight at Flexport, a freight-forwarding company.
“No one company can own the end-to-end vaccine supply chain. Collaboration will be critical,” Mr. Shah said.
Throughout transportation, pharmaceutical companies and logistics providers will need to ensure doses are kept in a very tight temperature range to prevent the vaccine from becoming ineffective. That requires the use of specialized refrigerated containers and handling procedures at all times.
The drugmakers also need contingency plans in place in case all the preparations fall through.
“There’s storms, the plane doesn’t get off the ground, the truck gets involved in an accident,” said Mark Capofari, a supply-chain management lecturer at Penn State Lehigh Valley. “What plans do we have in place to get that product to put it back into cold storage?”
Drug makers will even need to be on guard against criminal groups that target high-value pharmaceutical goods, said William McLaury, an associate professor of supply-chain management at Rutgers Business School.
Finally, companies will have to be ready to adjust any part of the manufacturing and distribution process as scientists track responses to a vaccine—a process that will be as closely watched as the development of the drug itself, Mr. McLaury said.
“The supply chain is going to have to be on its toes,” he said.
Updated: 8-12-2020
Moderna Inks $1.5 Billion Coronavirus Vaccine Deal With U.S.
Under the U.S.’s latest deal for Covid-19 vaccines, Moderna agreed to supply 100 million doses.
Moderna Inc. said Tuesday it agreed to provide the U.S. government 100 million doses of its experimental coronavirus vaccine in exchange for more than $1.5 billion.
The drugmaker’s vaccine agreement is the latest with the U.S., which has already secured hundreds of millions of doses from other companies.
With the latest deal, the U.S. has agreed to spend more than $9 billion for the shots. It has also invested in vaccine research and development, as well as supplies like vials and syringes.
The government will provide Moderna’s vaccine to people in the U.S. free of charge and have the option to purchase up to an additional 400 million doses, according to Moderna.
“We appreciate the confidence of the U.S. government in our mRNA vaccine platform and the continued support,” Moderna Chief Executive Stéphane Bancel said in a statement.
The value of the agreement implies a price of about $15-per-dose, which appears to be lower than the price range of $32 to $37 per dose that Cambridge, Mass.-based Moderna said last week was part of small-supply government contracts.
Vaccines are seen by public health officials as the best way to curb the spread of the new coronavirus, yet their cost and price have emerged as important questions as they move forward in advanced stages of development.
“For Operation Warp Speed, we are assembling a broad portfolio of vaccines to increase the odds that we will have at least one safe, effective vaccine as soon as the end of this year,” U.S. Department of Health and Human Services Secretary Alex Azar said in a statement.
Moderna’s vaccine is among the most advanced in development. It is being evaluated in a 30,000-person late-stage study. Public health officials have said they may learn in November or December whether the Moderna vaccine works.
The vaccine, which is administered to patients in two doses, produced an immune response in the 45 healthy volunteers evaluated in an early-stage study and was generally safe and well-tolerated, according to results published last month by the New England Journal of Medicine.
Moderna has previously said its vaccine could get emergency approval in the fall if it proves to work safely in late-stage clinical trials, and has begun ramping up manufacturing in advance.
Moderna has received about $950 million in U.S. funding for the development and testing of its vaccine, which the company codesigned with the National Institutes of Health. Taking this funding into account, Moderna’s vaccine price per dose jumps to about $25, according to analysts at Morgan Stanley.
In recent weeks, the U.S. has struck deals with most of the companies developing the most advanced vaccines. Johnson & Johnson last week said it agreed to provide 100 million doses of its coronavirus vaccine for use in the U.S., in exchange for more than $1 billion from the federal government, implying a per-dose price of about $10.
Under a deal announced last month, the U.S. agreed to pay Pfizer Inc. and BioNTech SE $1.95 billion for 100 million doses, which suggests a price of $19.50 a dose and $39 for two doses. AstraZeneca PLC, meanwhile, agreed to provide 300 million doses to the U.S. for $1.2 billion, suggesting $4 a dose for the vaccine it co-developed with University of Oxford researchers.
Moderna has said it plans to make at least 500 million doses a year starting in 2021, and possibly as many as a billion.
Moderna’s vaccine uses a novel gene-based technology known as messenger RNA, which carries instructions from DNA to the body’s cells to make certain proteins. An mRNA vaccine has never been approved to prevent any infectious disease, and Moderna has never received approval for any drugs or vaccines.
Updated: 8-24-2020
FDA Authorizes Convalescent Plasma For Covid-19 Use
The emergency-use authorization comes after preliminary studies supported the benefits of the antibody-rich plasma taken from recovered Covid-19 patients
The Food and Drug Administration authorized use of convalescent plasma, the antibody-rich blood component taken from recovered Covid-19 patients, for the treatment of serious coronavirus cases.
The agency’s action Sunday, called an emergency-use authorization, permits use of the treatment on hospitalized Covid-19 patients. The Wall Street Journal reported last month the move was coming.
For Covid-19 patients and the doctors who treat them, the designation opens up the possibility for faster and easier access to a promising treatment. But the FDA said more clinical studies are necessary for definitive proof of the therapy’s effectiveness.
Doctors have been looking for validated coronavirus treatments. Until now, only one other drug, remdesivir from Gilead Sciences Inc., has the FDA’s emergency-use OK.
Certain seriously ill Covid-19 patients treated with plasma containing high levels of antibodies appeared to benefit more than those who received low levels of antibodies, said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
Hospitalized patients who received the plasma within three days of diagnosis, are under the age of 80 and not on mechanical ventilation, benefited the most, with a 35% improvement in survival 30 days after receiving the transfusion compared with patients who got plasma with low antibody levels, according to Dr. Marks.
Convalescent plasma has been seen as a way to help people fight the disease and a bridge while other treatments are under development. The emergency-use authorization doesn’t alleviate the need for a vaccine or for therapies known as monoclonal antibodies that could stave off infection or at least reduce the seriousness of one.
President Trump, at a White House news conference Sunday, described the FDA’s move as a breakthrough that makes the product far more widely available than it has been. He also took credit for the timing, saying his administration removed barriers delaying action.
FDA Commissioner Stephen Hahn said the authorization falls short of a full approval and the agency will evaluate more evidence.
Emergency-use authorization waives some regulatory requirements involved in using products during public health emergencies that aren’t yet FDA-approved.
Mr. Trump, who has spread messages attacking scientists including National Institute of Allergy and Infectious Diseases Director Anthony Fauci, criticized the FDA in a tweet yesterday, saying the agency was impeding efforts to roll out coronavirus vaccines and drugs for political reasons.
Mr. Hahn was nominated by Mr. Trump. The vaccines and drugs furthest along in development are moving at a remarkable speed, industry and government officials say. Several are now in late-stage testing, which the agency typically requires to assure they work safely.
White House chief of staff Mark Meadows suggested on the show “Fox News Sunday” the FDA could have moved faster to issue the emergency-use authorization.
Democrats criticized the president and his administration for politicizing the FDA. “The FDA must approve drugs or vaccines based on their safety and effectiveness—NOT political pressure from the White House,” House Speaker Nancy Pelosi said in a tweet on Saturday.
There is a history of using convalescent plasma to treat people during viral outbreaks, such as the 1918 influenza pandemic and the 2014 Ebola outbreak in West Africa. But there is still considerable debate within the scientific and medical establishment about drawing conclusions about the benefits of a drug tested outside a rigorous clinical trial.
The expanded access study was set up in the early days of the pandemic to allow easier access to plasma and ensure it was safe, not to establish if the drug was effective. Emergency use authorization may also make it difficult to enroll hospitalized patients in clinical trials.
The agency had been moving toward greenlighting convalescent plasma for weeks, according to people familiar with the matter. At the request of the National Institutes of Health, last week, it examined new data from the Mayo Clinic and colleagues who have been evaluating the treatment in thousands of Covid-19 patients, according to a person familiar with the matter.
The FDA’s action is based primarily on analyses of recent data from a large expanded-access program sponsored by the agency and led by the Mayo Clinic in Rochester, Minn. More than 72,000 Covid-19 patients have been transfused to date through the program, though the FDA relied on data from a fewer number of patients for its analysis.
The Mayo Clinic researchers reported, in a paper posted on a public server earlier this month, that patients treated with plasma with high levels of antibodies within three days of diagnosis had a mortality rate of 8.7% at seven days after the transfusion, compared with a mortality rate of 11.9% for patients who got plasma with low levels of antibodies at four days or more after diagnosis.
The analysis has limitations. It hasn’t been published in a journal or subjected to peer review. Also, expanded-access programs aren’t as rigorous as a clinical trial, which compares how subjects who got the study drug did at random with subjects who received a placebo or the standard of care.
Given the shortcomings, Mayo Clinic researchers can’t definitively say whether plasma improves outcomes because every patient in the study receives it. Rather, they have reported signs the treatment might be working in certain patients.
Other studies also have indicated that patients receiving high-antibody plasma early in the illness appear to benefit.
Researchers involved in a continuing study of more than 300 Covid-19 patients treated with convalescent plasma at Houston Methodist published a study earlier this month in the American Journal of Pathology finding those who received high-antibody plasma within 72 hours after hospitalization were more likely to survive than similar patients not treated with plasma.
The FDA’s Dr. Marks said the data used for the emergency authorization are preliminary and the agency will consider updating the authorization as more information is available.
Mr. Trump previously touted two malaria drugs, hydroxychloroquine and chloroquine, to be used in Covid-19 patients. The FDA subsequently gave the products an emergency-use authorization, then revoked it in June after concluding that the two related drugs were unlikely to be effective in treating the virus in hospitalized patients outside of a clinical trial.
Updated: 8-31-2020
Covid-19 Vaccines: What’s Coming and When?
As countries and companies race to produce a safe and effective coronavirus vaccine, here’s a guide to the front-runners.
Some 170 Covid-19 vaccines are in development around the world, according to the World Health Organization, each one promising to protect people from the deadly coronavirus and allow them to go back to work and school.
Now, a handful are starting or nearing the final stage of testing. Depending on the results, some companies say their vaccines could be greenlighted for use as soon as this year.
How Far Along The Candidate Vaccines Are
Each line represents one vaccine candidate. Colors represent the technologies each vaccine uses to produce an immune response in the body. The length of the line represents the stage of testing.
The Front-Runners
Among the first vaccine candidates to start the final round of testing is one developed by the University of Oxford and AstraZeneca AZN 0.56% PLC. Also far along are experimental shots from Pfizer Inc. PFE -0.32% and its partner BioNTech SE, BNTX -4.30% as well as Moderna Inc.
China National Pharmaceutical Group Co., or Sinopharm, has a vaccine in Phase 3. A vaccine from another Chinese company, CanSino Biologics, is expected to begin the pivotal testing soon. But remember, many vaccines that show promise in early testing fail during the final round.
The Oxford/AstraZeneca vaccine is designed to provide protection by delivering into a person’s cells the genetic code for the spikes protruding from the new coronavirus. Then the cells can produce the spike proteins, generating an immune response that would be able to fight off the coronavirus. Delivering those genetic instructions is a weakened, harmless version of a virus that causes the common cold in chimpanzees.
In early testing, the vaccine successfully produced immune responses in humans with only minor side effects. A Phase 3 trial enrolling 30,000 subjects in the U.S. began in August. Other late-stage trials are under way with several thousand volunteers in the U.K., Brazil and South Africa.
The Moderna vaccine also uses a gene-based technology to provoke an immune response, though the code it delivers takes the form of messenger RNA. Those molecules, commonly referred to as mRNA, are the body’s molecular couriers ferrying DNA instructions for making proteins. The vaccine delivers to cells mRNA for making the coronavirus’s spike protein.
Moderna and the U.S. National Institute of Allergy and Infectious Diseases are testing a two-dose shot. It was the first candidate to enter human testing in the U.S. The vaccine produced an immune response in early-stage testing and was generally well-tolerated, with minor side effects observed in test subjects.
Final-stage testing is under way in the U.S. with a 30,000-person trial that could yield interim results in the fall. An mRNA vaccine has never been approved for any disease.
U.S.-based Novavax Inc. is making a vaccine that consists of two shots given 21 days apart that deliver proteins resembling the spike jutting out from the new coronavirus. Researchers hope the proteins will trigger the production of antibodies and immune cells that can fight off the coronavirus.
The shots also contain a component, called an adjuvant, to boost the immune response. In Phase 1 testing, the vaccine was generally well-tolerated and produced promising numbers of antibodies. Phase 2 testing began in August, and the company has said Phase 3 could start in September.
Updated: 10-11-2020
Antibody Drugs Touted by Trump Could Be Next To Get Authorized For Covid-19
If the drugs are greenlighted, expected heavy demand is likely to surpass limited initial supplies from Regeneron and Lilly.
President Trump’s endorsement of an experimental Covid-19 drug from Regeneron Pharmaceuticals Inc. has raised expectations for a type of medicine that could be authorized for public use within weeks or even days.
Regeneron is racing against rival Eli Lilly & Co. to bring the first monoclonal antibody drug on the market to treat Covid-19 patients who aren’t sick enough to be hospitalized. Both companies said Wednesday they had asked the U.S. Food and Drug Administration to authorize use, and they had already made tens of thousands of doses for patients.
If greenlighted, the shots would begin to fill a big hole in the Covid-19 medicine chest for treatment of early and less-severe cases. After more testing, they could offer temporary protection against infections until vaccines arrive. But supply is unlikely to meet demand until next year at the earliest.
Monoclonal antibodies, which are also used to treat cancer and other diseases, take a page from the body’s own soldiers for fighting pathogens. Both Lilly and Regeneron researchers derived their drugs from naturally occurring Covid-19 antibodies.
Each of the companies has released encouraging data from early-stage clinical trials showing their drugs helped reduce coronavirus levels and improve symptoms in patients not sick enough to be hospitalized. Lilly and Regeneron have already started studies to see if their antibody drugs can prevent Covid-19.
Given the urgent need, the companies asked the FDA to use a special review process reserved for emergencies such as pandemics and authorize use before the therapies have finished all the stages of testing normally required for approval.
The FDA could issue the go-ahead for the monoclonal antibody drugs as soon as Friday, SVB Leerink analyst Geoffrey Porges said. The agency could feel comfortable making a quick decision, he said, because it is already familiar with the way antibody drugs work, having approved many of them over the years to treat other diseases.
The FDA declined to comment about its review of the applications or the timing of a decision.
The agency has granted emergency-use authorizations for two therapies for hospitalized Covid-19 patients, the antiviral remdesivir from Gilead Sciences Inc. and antibody-rich convalescent plasma taken from recovered coronavirus patients. An antibody drug could be the next agent to be permitted for use.
Last Friday, Mr. Trump was given a high dose of Regeneron’s drug, code-named REGN-COV2, under a program that allows patients to take experimental drugs outside of clinical trials. He was later given the antiviral drug remdesivir, which the FDA authorized in May, and the already approved steroid dexamethasone.
Mr. Trump’s doctors have said he is symptom free. In a video released late Wednesday, the president attributed his return to health to Regeneron’s drug.
“They gave me Regeneron, and it was like, unbelievable. I felt good immediately,” he said. Independent doctors have cautioned against singling out any specific factor for Mr. Trump’s feeling better, or even concluding he has recovered, given the unpredictable course of the disease and various care he received.
“The president’s team of medical consultants and advisers all thought that for a person in his position, they saw the benefit-risk equation, and they thought that this might be a reasonable thing to do,” George Yancopoulos, Regeneron president and chief scientific officer, said in a recent interview. “I think it means that there’s going to be a lot of other people who come to that conclusion.”
Mr. Trump also said he encouraged the authorization of antibody drugs and would make sure patients wouldn’t have to pay for them.
Companies normally don’t request the FDA’s clearance of a drug until they have discussed the matter with the agency and believe they have the kind of data that could secure a regulatory green light. The agency will, nevertheless, sometimes refuse to approve a drug after reviewing the application, or ask for more testing.
Administration officials could take the reins from the agency, as they did in 2011 when Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to make an emergency contraceptive broadly available, including to young teenagers.
Regeneron and Lilly said they didn’t know how long the FDA would take to review the requests for authorization. Lilly has been working with FDA reviewers for several months at a fast pace, company executives said, and they expect that clip to continue.
Both companies said they have been making doses. Regeneron said it has enough supply to treat 50,000 patients right now, and for a total 300,000 patients within a few months. Lilly said it would have 100,000 doses by the end of this month, and as many as a million by year’s end.
The supply probably won’t be enough to give the drugs to everyone who could take it. More than 44,000 Americans were diagnosed with Covid-19 on average each day over the past week, according to Nephron Research, a health-care-focused investment research firm.
“We just know that supply isn’t going to meet the needs of people around the world” initially, Lilly’s chief scientific officer, Daniel Skovronsky, said in an interview. Lilly executives said the federal government would decide who should get initial supplies, but the company suggested people over age 65 years or who are obese should be candidates because they are at higher risk of worsening to severe Covid-19.
Regeneron would try to make sure its drug would be “distributed fairly and equitably to the patients most in need” if it is authorized, a company spokeswoman said.
To boost production, Lilly in September signed a collaboration with Amgen Inc. in September to help manufacture Lilly antibody therapies. Roche Holding AG agreed in August to help make and distribute Regeneron’s antibody product.
Manufacturing monoclonal antibodies is more complicated, expensive and time-consuming than making traditional pills. Antibody drugs are grown in living cells fed various nutrients to cultivate their development. Regeneron said it takes two months to make its Covid-19 drug.
Neither Lilly nor Regeneron has given a price for their therapies, though both have said they expect patients will be able to get the drugs with no or very low out-of-pocket costs. Regeneron said the U.S. government would distribute the first 300,000 doses of its drug free, because it helped fund the drug’s development.
Antibody drugs for cancer, rheumatoid arthritis and other diseases can cost tens of thousands of dollars a year or more.
Lilly Chief Executive David Ricks said in a call with reporters Wednesday that the company would consider the special situation created by the pandemic in pricing its Covid-19 drug, though it must also account for its heavy investment.
It will be hard for the drugmakers to set too high a price, SVB Leerink’s Mr. Porges said, because most patients who get the drug probably wouldn’t have developed a severe case anyway.
“If you treat 100 individuals with an expensive IV infusion to keep five from going into the hospital, it’s not a very compelling value proposition,” he said.
Mr. Trump’s endorsement of the antibody drugs, and his promise to make them available free, will likely cause many infected patients to seek prescriptions even if they aren’t showing any symptoms, Mr. Porges said. He expects insurers to triage patients by setting criteria for who can receive the medications, such as whether they have symptoms and risk factors.
AstraZeneca PLC said Friday that it will start testing its monoclonal antibody drug in a Phase 3, or final-stage, clinical trials in the coming weeks. The drug, code-named AZD7442, combines two antibodies derived from patients who recovered after being infected by the new coronavirus.
AstraZeneca said one study will enroll 5,000 healthy volunteers to test if the drug can prevent infection for up to one year; the second study will test the drug’s effectiveness in preventing or ameliorating symptoms in 1,100 patients who have been exposed to the virus.
The U.S. government is supporting the development of AstraZeneca’s drug with $486 million. The U.S. is entitled to receive up to 100,000 doses of the drug toward the end of 2020, and the option to acquire an additional one million doses in 2021 under a separate agreement, AstraZeneca said.
Updated: 4-1-2021
Vaccine Trickle Becomes Torrent As U.S. Eligibility Rules Widen
It’s taking some effort and some patience. But just as eligibility is opening to millions of people across the U.S. after months of cutthroat competition to find Covid-19 shots, vaccines are starting to stream into people’s arms.
Becky Jacobsen, 41, was ready to drive as long as an hour for a shot as soon as Connecticut made all adults eligible on Thursday. A friend stayed up late to snatch an appointment at a CVS Health Corp. drugstore just 6 miles from Jacobsen’s home in Windsor.
“Another friend is looking for my husband,” the mother of five said by phone as children shouted in the background. “It’s distance-learning day and I’ve got to focus on making sure the kids aren’t on YouTube when they’re supposed to be on Google Classroom.”
President Joe Biden staked his bid on an effective battle against the coronavirus that would center around mitigation measures and assisting states with the swift dispersal of vaccines.
States are offering shots to millions of people who want to return to life as it was before Covid, and officials in charge are reporting that the campaign is rounding into form.
The progress offers hope that most adults will be vaccinated this summer before attention shifts to children. Positive data from partners Pfizer Inc. and BioNTech SE this week could position 12- to 15-year-olds for a shot before the next school year.
Nearly 100 million people, or almost a third of the U.S. population, have already received at least one shot, according to data from the Centers for Disease Control and Prevention. The U.S. has administered more doses than any other nation, though it ranks seventh in terms of the percentage of the population with at least one dose, according to the Bloomberg Vaccine Tracker.
Floodgates Open
Nearly half of U.S. states will have opened vaccination to everyone 16 and older by the end of this week. That will rise to about three-quarters, or 35 states, by the end of next week.
Millions more doses are being distributed every week. Moderna Inc. and Pfizer-BioNTech are each on track to deliver enough shots to vaccinate 100 million people in the U.S. by the end of May, according to the Bloomberg Vaccine Tracker.
While some 15 million Johnson & Johnson shots were affected by a production issue in Baltimore, that’s not expected to have an effect on Biden’s expectation that the U.S. will have enough vaccine for all adults in May, according to people familiar with the matter. Likewise, delays in the U.S. clearance of AstraZeneca Plc’s vaccine, which is still the subject of side-effect concerns, probably won’t affect the U.S. campaign.
The floodgates are beginning to open in Los Angeles where residents have begged, borrowed and stolen to get appointments. Some people expressed surprise on social media about how simple it’s become.
“I just made an appointment for Wed.,” Matt Oswalt, a comedy writer and photographer wrote on Twitter this week. “Took 2 minutes to book it. Easy, they have tons of slots open.”
‘He Curses’
Not everyone is having as much luck. Judith Romano from Ashland, Massachusetts, got her first of two Pfizer shots on Thursday at a Boston clinic, but said her husband, a grocery clerk in his late 50s, has still been unable to schedule one through the state sign-up system.
hey anyone in LA who meets the Covid vaccine eligibility and is having trouble getting the shot.
Go to this website – I just made an appointment for Wed. Took 2 minutes to book it. Easy, they have tons of slots open. Dodger Stadium, valley,…everywherehttps://t.co/SJOv0SSYcZ— Matt Oswalt (@MattOswaltVA) March 30, 2021
“He’s been trying, trying, trying trying,” she said. “People say, ‘Oh, you’ve got to get on at like 4 a.m. And just wait.’ But that’s silly. Why should you have to do that?”
Each time her husband fails to get a slot, “he curses,” she said.
Alabama remains one of the slowest states in the U.S. for getting vaccines into arms. But its performance has been improving, particularly in poor minority areas that weren’t getting doses in the campaign’s first several weeks.
Alabama Regional Medical Services, a low-income clinic in North Birmingham, didn’t get a single dose through the end of February. In March, though, it got nearly 5,000 from the state, said Chris Mosley, a spokesman for the clinic.
Spring Flowers
The shift was partly because the state eased guidelines to include essential workers – many of them minorities – but largely because of coordination with community groups, Mosley said.
“Now vaccination sites are sprouting up like spring flowers,” he said. “It’s become almost a friendly competition, to see who can give out the most.”
The region has two mass-vaccination sites now, one inside an airport. People have also learned to be patient, Mosley said.
When the Alabama clinic drew a crowd of 350 for its first 200 doses, 11 disabled men living in a nearby group home were among those turned away. They got their shots last week, “after a year of being shut in, no place to go,” Mosley said. That night, their caregiver called Mosley and held the phone up.
“Thank you,” the 11 shouted in unison.
Proceeding Cautiously
Some states are still proceeding cautiously. New Jersey has so far held firm to its May 1 expansion date despite neighboring New York qualifying all adults on April 6. New Jersey Governor Phil Murphy acknowledged that the state’s vaccination-scheduling website will be strained.
“When you add a new group of people you add, at least temporarily, to the supply-demand imbalance,” he said Wednesday in a briefing, “but I’m confident that the system will hold up well.”
Pennsylvania scheduled universal eligibility for April 19, yet Philadelphia, which receives its own supply, plans to stick to its May 1 date, James Garrow, a spokesman for the city’s public health department, said in an email.
Alabama health officials are in conversations with the governor’s office about whether the state can offer vaccination to every adult before May 1, the deadline Biden set, assistant state health officer Karen Landers said.
Nerve-Wracking
“We do recognize we could have bottlenecks, but at the same time we are anxious for our citizens that want the vaccine to be able to receive it,” she said in an interview.
People need to understand that supply won’t magically increase, Washington state Secretary of Health Umair Shah said during a briefing Thursday. Some are learning that firsthand.
Jacobsen felt the pressure when Connecticut made her and every other adult able to get vaccinated. She couldn’t stay glued to her phone because she was busy wrangling five kids between the ages of 4 and 13, who could also take some time to get vaccinated.
“It feels chaotic and nerve-wracking,” Jacobsen said. “If you get an appointment you feel lucky. I don’t doubt everyone will get it at some point but it’s kind of like a mad rush for food in a soup line or something. I’m trying to think of the best way to describe it. It’s just unnerving.”
Updated: 4-24-2021
Vaccine Shortage Holds Emerging Markets Back From Global Rally
Developing stocks are lagging the rest of the world at a time when they should be tearing ahead.
As the pandemic spins out of control from India to Argentina, the divisions between emerging and developed markets are deepening.
Developing-nation stocks have lagged the rest of the world since the middle of March partly on concern that vaccine shortages and delays will slow economic growth. Investors pulled $1.3 billion out of emerging-market equity funds in the week ending April 21, the most in more than three months.
It’s one more data point that shows how this pandemic has rewritten the investing playbook. Historically, emerging markets were viewed as a way to ride a global economic expansion, but this time around, it’s the developed world that’s bouncing back the fastest.
U.S. and European stocks are near all-time highs, and JPMorgan Chase & Co warned last week that the U.S. economy will outpace emerging markets at an “unprecedented” rate in the second quarter due to slow vaccine rollouts in the developing world.
“Not only do we have a much slower vaccination program across emerging markets, but worries over debt loads, external vulnerabilities, fiscal prudence, inflation and currency stability will hamper a much stronger recovery post-pandemic,” said Mohammed Elmi, a portfolio manager at Federated Hermes in London. He recommends rotating into countries and assets that benefit from U.S. growth via trade links or exports, such as Mexico.
The MSCI Inc. gauge of developing-world shares has risen just under 5% so far this year, about half as much as the gain in a similar index of developed-market equities. Compare that to 2009, the year the global economy began to recover from the financial crisis, when emerging stocks soared 74%, almost three times more than their developed peers.
Among the factors driving the divergence lies a shortage of vaccines and their lopsided distribution that’s paving the way for a re-opening of Western economies even as major emerging-market economies struggle with new waves of infections.
More than a third of Covid-19 inoculations have gone to people in the world’s 27 wealthiest countries, which account for just 11% of the global population, Bloomberg’s Vaccine Tracker shows.
The disparity is particularly extreme in India, which is home to 18% of the global population and the epicenter of one of the most deadly outbreaks. Indian equity funds suffered their biggest outflow in more than a year in the week ending April 21, according to EPFR Global data, while the rupee has slumped 3.5% in the past month.
“This is likely to weigh on market sentiment in the near term,” said Tai Hui, chief Asia market strategist at JPMorgan Asset Management, who says investing in emerging-markets outside of Asia “will require a bit more patience for the pandemic to come under control.” For now, he recommends inventors concentrate their allocation in the U.S. and China.
Some investors are looking past the latest surge in cases, betting that vaccines will proliferate in the developing world. The fact that Europe is also beginning to recover should boost exports and add to inflows from tourism.
Current market valuations “leave a lot more room for upside in EM and for disappointment in the U.S,” according to Morgan Harting, a New York-based money manager at AllianceBernstein.
The slow pace of vaccinations in the developing world is adding to an already challenging backdrop for many emerging markets, which have had to navigate higher U.S. Treasury yields even as the cost of combating the pandemic piles pressure on their own finances.
For Jon Harrison, London-based managing director for EM macro strategy at TS Lombard, the prospect of the Federal Reserve tapering its asset purchases could also lift the dollar, putting an end to a key tailwind for the asset class this year. “In the absence of a return to a softer dollar the external backdrop for emerging-market economies is set to become more challenging” he said.
Updated: 9-13-2021
Covid-19 Deaths In Delta Surge Trend Younger In U.S.
Vaccines have shielded older people from the worst outcomes, leaving younger people who haven’t gotten shots at risk.
A surge in Covid-19 deaths caused by the highly contagious Delta variant is hitting working-age people hard while highlighting the risks for people who remain unvaccinated.
Federal data show Covid-19 deaths among people under 55 have roughly matched highs near 1,800 a week set during last winter’s surge. These data show weekly tallies for overall Covid-19 deaths, meanwhile, remain well under half of the pandemic peak near 26,000 reached in January.
The Delta-driven Covid-19 surge is the first major case surge to spread through a partially vaccinated U.S. population. High vaccination rates among the elderly, who are more vulnerable to severe Covid-19 outcomes, are restraining the overall increase in deaths, some researchers say.
The change is shifting a larger share of deaths to younger populations with lower vaccination rates, underscoring the need to get more people inoculated to curb the pandemic, they say.
“We don’t want anyone to die from a vaccine-preventable disease,” said Samuel Scarpino, managing director of Pathogen Surveillance at the Rockefeller Foundation’s Pandemic Prevention Institute.
The seven-day average for newly reported Covid-19 deaths each day recently eclipsed 1,600, up from an average that briefly moved below 220 a day in early July. With roughly 660,000 known Covid-19 deaths to date, the U.S. is on track to soon top the estimated 675,000 deaths that the Centers for Disease Control and Prevention has linked to the 1918-19 flu pandemic.
Deaths have been concentrated among the unvaccinated, federal data show. The CDC released studies on Friday showing that unvaccinated Americans were 4.6 times as likely to be infected, 10 times as likely to be hospitalized and 11 times as likely to die.
At Tampa General Hospital, about 90% of recent Covid-19 patients were unvaccinated, said Peggy Duggan, chief medical officer at the facility, which is one of Florida’s largest hospitals with more than 1,000 beds. Many patients who did get the shots have compromised immune systems due to organ transplants or cancer treatment, Dr. Duggan said.
Tampa General’s recent Covid-19 patients in intensive care were 46 years old on average, far below the average during prior surges when vulnerable seniors were often hospitalized, Dr. Duggan said. The hospital’s death rate for Covid-19 patients hasn’t changed, sticking around 7%.
“These are working people, they’re people with families and children they’re still raising,” Dr. Duggan said.
Younger age groups have represented a growing share of deaths since vaccines became available, a trend that has continued into the summer’s Delta surge.
Age is a major risk factor for people with Covid-19. People in their 30s are four times as likely to die from infections as people ages 18 to 29, according to the CDC. For people ages 75 to 84, the risk of death is 220 times as high.
Older Americans still account for the most Covid-19 deaths, but their higher vaccination rates have helped hold down the numbers. About 54% of the overall U.S. population and 63% of eligible people ages 12 and above are fully vaccinated, while the average among nursing homes is 84% for their residents, federal data show.
This is yielding benefits in places hit hardest by deadly Covid-19 waves earlier in the pandemic.
While the Delta surge set off nursing-home outbreaks again, and workers who chose not to get the vaccine prompted employers and officials to issue mandates for these facilities, residents’ deaths haven’t reached the levels seen earlier in the pandemic.
Despite gains in protecting seniors, the Delta surge has presented major risks to other groups. CDC data continue to show that, compared with non-Hispanic whites, Black and Hispanic people face almost three times the risk of hospitalization and more than twice the risk of death.
The rates among Native Americans are even higher. Rates among Asian people are comparable to those of non-Hispanic whites. The disparities stem from factors including pre-existing health conditions, access to healthcare and occupational exposure, public-health experts say.
A measure of daily Covid-19 deaths divided into new hospitalizations from two weeks earlier—covering a gap that clinicians say captures the time it takes for many deaths to occur—shows some turbulence since Delta began driving up cases this summer.
This metric counts all Covid-19 deaths, including the roughly one-third that occur outside hospitals, meaning it doesn’t precisely reflect the rate at which hospitalized patients die.
Measuring deaths against hospitalizations shows a decline in deaths after the mass vaccination effort began, reflecting what some health-experts say is more exposure among younger people and improvements in care, but that decline stopped in the spring.
Estimating the overall share of Covid-19 cases that become deadly is difficult, because testing captures only a portion of actual cases. A measure known as the case-fatality ratio, which looks at the number of known cases that become known deaths, appears to be heading lower, some public-experts said.
But because deaths tend to lag three to five weeks behind cases, the death toll may yet grow even as new cases are leveling off. The concentration of those fatalities among unvaccinated people demonstrates how important the shots are to ending the pandemic, said Jodie Guest, vice chair of the epidemiology department at Emory University’s Rollins School of Public Health.
“We cannot accept this as our endemic level, and we have the tools to keep this from being our endemic level,” Dr. Guest said.
Updated: 9-15-2021
Is the Mu Variant More Dangerous Than Delta?
Even as the world combats the fast-spreading delta virus variant, a new strain, mu, has emerged.
Here’s what you need to know.
The highly infectious delta variant has sparked a new wave of Covid-19 cases and deaths worldwide in recent months, hampering reopenings and slowing the economic recovery. Now comes word of a new variant, mu, which has taken hold in some countries.
Sam Fazeli, a Bloomberg Opinion contributor who covers the pharmaceutical industry for Bloomberg Intelligence, answers questions about the emergence of this new variant and its potential danger.
How Worried Should We Be About The mu Variant?
Not much, at least for now. Every mutation has the theoretical chance to make the virus more “fit” in its ability to infect, copy itself and spread. What makes a mutation or group of mutations “of interest” or “concern” to scientists is when it emerges in a large enough population and is seen multiple times. The mu variant is the dominant one in Colombia, where it displaced the gamma variant.
Even though assessing virus variants in Colombia is hard given the low number of genomic sequences analyzed, there has been an increase more recently in the delta variant there. Looking at other countries with more sequencing efforts, such as Austria, Chile, Mexico and Spain, it is clear that the mu variant is not as “fit” as delta, with the latter squeezing all other variants out.
What Causes A Virus Strain To Be More Resistant To Vaccine-Driven Immunity?
Virus versus host is a constant battle. The virus infects an individual, who then raises an immune reaction against it — initially antibodies, followed by cellular immunity, i.e., “T-cells” and then “immune memory.”
Random mutations in the virus then potentially give it the ability to become less sensitive to the initial antibodies, making it more able to infect people who had been previously infected. A similar situation is possible in people who are immunized using a vaccine.
But the immune system also evolves and produces an array of antibodies that allow it to recognize new mutants or variants. The mutations that lead to the virus being able to evade vaccine-induced antibodies tend to occur at regions of the “spike” protein to which antibodies bind and stop an infection.
The most relevant of these has been the mutation at the so-called 484 position of the spike protein, which has been seen in the beta, gamma, kappa, eta, iota and mu variants.
What Is It About Delta That’s Made It So Dominant Even In Competition With Variants That Have More Mutations?
This brings us to an important point. While it is not known exactly why the delta variant is so much more infectious than other variants, it has been assumed that it may have something to do with a key mutation in spike position 681, which has also been seen in the mu, kappa and alpha variants.
So it’s clearly not just about this one mutation, given that delta has so far taken over wherever it has been introduced. And delta’s ability to infect more quickly and multiply faster than other variants also affects its ability to infect vaccinated people.
If there aren’t enough antibodies at the sites of infection, i.e., the nose or throat, then the virus will have a clearer path – and with delta you see a rapid rise in viral particles.
So an infection happens not just because the virus has become better at evading vaccine-induced antibodies, or those from a previous infection, but also because the time course of an initial infection is too fast for the immune system to react, especially in those who were vaccinated several months ago and are experiencing the expected waning of antibody levels.
Are We Doing Enough Tracking To Ensure We Catch More Concerning New Variants?
No. We need a lot more genomic sequencing than we have at the moment. There is a high level of sequencing in some countries such as the U.K. and Denmark, but others are still too low on a per-capita basis. But it’s not just about the numbers of sequences.
It’s also about how quickly they are reported. For example, based on data from CoVariants.org, the U.K. filed close to 50,000 sequences to GISAID (a global effort for sharing and tracking information on viruses) between Aug. 23 and Sept. 6, while France submitted a meager 124 sequences during the same period, essentially suggesting that it doesn’t truly know what is happening in the country.
Some People Seem To Believe That Vaccination May Drive The Virus To Become More Evasive, As Antibiotics Can Do For Bacteria. But That’s Incorrect, Right?
I hear this often, and it makes me cringe. The situation with vaccines is much different when compared with antibiotics. Vaccines and natural infection generate an immune response at two different levels — antibodies and T-cells — against many different sites, known as epitopes, on the virus. T-cells can also target epitopes different from those targeted by antibodies.
The immune response then evolves naturally over time. So even though immune-evading viral variants have developed and will continue to develop, the situation is not analogous to antibiotic resistance. And you can boost immunity with additional shots of vaccine.
Will Vaccines Work Against Mu?
Remember, vaccines are not designed to prevent infections, they are intended to prevent disease.
So as long as our vaccine-induced immunity is able to prevent severe disease, hospitalization and death, then we are mostly fine. We know that mu can evade antibodies at about the same level as the most evasive of variants to date such as beta.
But that just means that it can potentially infect people who are vaccinated (just as delta can), bypassing their initial antibody protection in the nose and throat. It says very little about whether it can cause disease, given that the “memory” antibody response kicks in to produce many antibodies, and cellular immunity (T-cells) comes into its critical role.
How Should Public Health Authorities Draw The Line Between Alerting Folks To Threats And Avoiding Panic-Mongering?
I think they should keep “variants of interest” to themselves. I don’t see what purpose it serves to let the world at large know that there is a variant of interest, which in most cases ends up not amounting to much in terms of increased risks or dangers.
Updated: 9-19-2021
California Has U.S.’s Lowest Infection Rate
California Governor Gavin Newsom, who survived a recall election partly on his handling of the pandemic, tweeted on Saturday that California now has the lowest infection rate among U.S. states.
The most populous U.S. state had rate of 113 cases per 100,000 people, over the last seven days, according to the latest data from the Centers for Disease Control and Prevention. The next closest state was Connecticut, almost 126 cases per 100,000. Florida had 353 per 100,000, CDC data show.
He also said that California had crossed the milestone of administering at least one dose to 70% of the population.
“Vaccines are how we end this pandemic,” he tweeted.
Updated: 9-27-2021
Unproven Covid Pills Could Be Biden’s Secret Weapon
Highly convenient oral drugs could be a pandemic game changer. With enough investment, the U.S. could make a whole lot of them for the world.
President Joe Biden unveiled big plans earlier this month for the U.S. fight against Covid-19, anchored by expansive vaccine mandates and a planned rollout of booster shots. On Wednesday, he turned his focus abroad, hosting a virtual vaccine summit with global leaders aimed at protecting the world. But there’s more he can do.
Three pharmaceutical giants — Merck & Co., Pfizer Inc. and Roche Holding AG — are in the late stages of testing oral antiviral drugs (Merck in partnership with Ridgeback Biotherapeutics LP and Roche with Atea Pharmaceuticals Inc.) that aim to block the Covid-19 virus from replicating.
If given early in the course of infection, these medicines have the potential to keep people from getting severely ill or ending up in the hospital. Because pills are relatively cheap to manufacture in large amounts and easy to distribute, expanded funding could make them a pandemic game changer.
The U.S. has already promised to buy a limited supply of Merck’s treatment and announced $3.2 billion in general antiviral investment in June.
But investing more in all three programs ahead of authorization could ensure ample supply of what may be very useful medicines to deploy as soon as regulators give the go-ahead.
It’s a strategy the country used earlier in the pandemic under the Trump administration, with deals that gave companies cash up front to build up inventories of vaccines and antibodies.
With these new oral treatments, Biden can follow the same playbook, only at a larger scale and with faster results. Data is due from key clinical trials of all three leading candidates in the next few months, and while failure could see money wasted, the potential rewards are worth the risk.
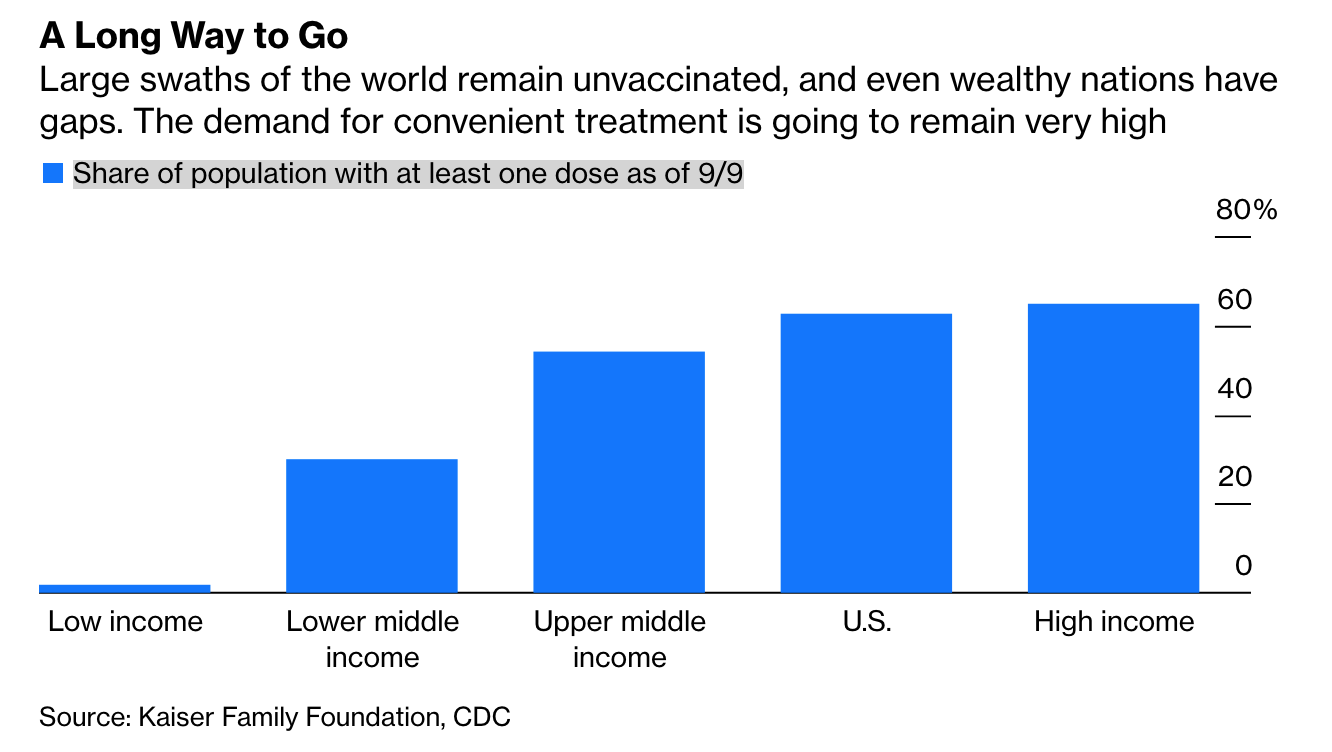
Compared to antibodies and vaccines, which are biologic products that are expensive to manufacture and can have burdensome storage or delivery requirements, these antivirals are refreshingly straightforward. Pills can be stamped out in large volumes using widely available equipment and dispensed through local pharmacies or clinics with no needles or freezers required.
A course of Merck’s pill will cost the U.S. a third of what it has paid for Regeneron Pharmaceuticals Inc.’s antibody therapy, if it proves successful. That’s even before accounting for the much higher cost of administering antibodies.
On the subject of antibodies, supplies remain inadequate in the U.S. and absent in many countries. As for vaccinations, as of Sept. 9, only 2% of people in low-income countries had received one dose. Fixing this is crucial, but supply chains are stretched, and additional capacity will take time to come on line. In that context, it’s crucial to also leverage the potential advantages of oral treatments.
The continuing popularity of ivermectin, a drug for parasitic diseases that hasn’t shown firm evidence of efficacy, makes it clear that there’s substantial demand for a convenient option that really works.
The relative ease, speed, and economy of pill production could result — with sufficient investment and trial success — in enough timely doses for the entire world. The biggest benefit of expansive supply would come in low-income nations otherwise left behind. Millions will remain vulnerable for months to come as they wait for vaccines, making treatments a crucial protective bridge.
And the value of an easily dispensed treatment that keeps people from getting severely ill is higher in places with weaker infrastructure. The key is to spend early, be ambitious, and focus on direct financing of global manufacturing capacity.
“This is a global tragedy,” Biden said at the vaccine summit. “And we’re not going to solve this crisis with half-measures and middle-of-the-road ambitions. We need to go big and do our part.”
He’s right. But he needs to think beyond the shot.
Updated: 10-1-2021
Merck Pill Intended To Treat Covid-19 Succeeds In Key Study
Drug jointly developed with Ridgeback Biotherapeutics cut risk of hospitalization or death by 50% in early look at progress.
Merck & Co. and its partner Ridgeback Biotherapeutics LP said their experimental Covid-19 pill helped prevent high-risk people early in the course of the disease in a pivotal study from becoming seriously ill and dying, a big step toward providing the pandemic’s first easy-to-use, at-home treatment.
The pill cut the risk of hospitalization or death in study subjects with mild to moderate Covid-19 by about 50%, the companies said Friday.
The drug, called molnupiravir, was performing so well in its late-stage trial that Merck and Ridgeback said they stopped enrolling subjects after discussions with the U.S. Food and Drug Administration.
The results put molnupiravir on track potentially to be authorized by the end of the year and to finally provide an option for doctors who have spent the pandemic seeking a drug that infected people could easily take at home to prevent them from becoming hospitalized.
Merck plans to ask the FDA to authorize the drug’s use in the coming weeks, Chief Executive Rob Davis said.
If cleared by regulators, the drug would be the first oral antiviral for Covid-19. Within five days of showing symptoms, people would take eight pills daily for five days, if they followed the same protocol used in the study.
Molnupiravir would become a kind of Tamiflu for Covid-19, a medication that can be dispensed to patients when they first develop symptoms, slowing the spread of the virus in the body and potentially preventing people from becoming seriously ill.
“The ability to take what is a devastating disease like Covid-19 and potentially make it a manageable situation through what is a very convenient round of administration, which is an oral pill you can take at home, has important implications for the ability to manage the ongoing pandemic,” Mr. Davis said.
Together with vaccines, the pill, if authorized, would help transform Covid-19 from a deadly pandemic disease that overwhelms hospitals to a treatable illness as it becomes endemic in the population. “This will help get to that endemic level and maintain that level,” said Gabor Kelen, director of the Department of Emergency Medicine at Johns Hopkins Medicine.
Doctors expressed concern, however, the pill’s promising results might encourage people who are vaccine hesitant to feel emboldened about not getting vaccinated.
“Vaccination is still, first and foremost, the most effective and least risky thing that you can do,” said John Mellors, chair of the Division of Infectious Diseases at the University of Pittsburgh Medical Center and the University of Pittsburgh.
Merck and Ridgeback reported the findings in a press release after an early look at results from the study, which is scheduled to finish in November. The results haven’t been published in a peer-reviewed scientific journal.
The companies said the rate of side effects in study subjects who got molnupiravir and those who got a placebo was similar, though they didn’t say in their news release whether the drug was found in the study to be safe. Mr. Davis, however, said he was confident in the drug’s safety profile.
The drug appeared effective against circulating Covid-19 variants, including highly contagious Delta, according to the companies.
The results position Kenilworth, N.J.-based Merck, one of the world’s biggest drugmakers, to play a larger role in responding to the pandemic after several setbacks. After its two experimental vaccines failed in testing, Merck agreed to help make Johnson & Johnson’s shot. Merck also halted development of an experimental drug it acquired through a $425 million purchase.
Molnupiravir could generate $10 billion in sales cumulatively through 2025, given potential for stockpiling and its potential for preventing Covid-19, which is still being studied, according to Daina Graybosch, an analyst at Leerink LLC. Merck shares rose more than 9% on the pill news.
Merck said it expects to produce 10 million courses of treatment by the end of the year, with more doses coming next year. If authorized, Merck would begin shipping doses fairly quickly, Mr. Davis said.
The U.S. has agreed to pay Merck $1.2 billion for 1.7 million courses of treatment, should regulators green light use of molnupiravir.
Merck said it plans to make the drug available world-wide and has licensing agreements with generic manufacturers to provide the drug to low-income countries, many of which have had difficulty accessing vaccines to slow the virus’s spread.
Vaccines are the main weapon fighting the pandemic, but doctors and health authorities have long looked for drugs that can help keep people who do get infected from developing severe illness and needing hospitalization.
Nearly two years into the pandemic, however, the drug options are limited. Remdesivir, from Gilead Sciences Inc., is the only antiviral fully approved by the FDA but is used only to treat hospitalized patients.
The FDA has cleared antibody drugs made by companies like Regeneron Pharmaceuticals Inc. and GlaxoSmithKline PLC and its partner Vir Biotechnology Inc. for people with mild to moderate Covid-19 who aren’t hospitalized.
The drugs reduced the risk of hospitalization or death by at least 70% in their trials, a higher efficacy rate than molnupiravir. Yet the antibody drugs are more complicated to administer than swallowing a pill, requiring an intravenous infusion, which initially slowed their uptake.
One of the more effective drugs against Covid-19, a steroid named dexamethasone, is for very sick patients.
“Even though on its face the pill may have shown less benefit than the monoclonal antibodies, because it will be so much easier to use, it will have a much bigger impact on the world stage,” said Daniel Kuritzkes, chief of the division of infectious diseases at Brigham and Women’s Hospital in Boston.
Dr. Mellors said the companies’ results, while encouraging, left him with questions as to how effective the drug is in vaccinated people with breakthrough infections and how its effectiveness compares with monoclonal antibodies. “We need to see the full data set,” he said. He said it would also be important to study the drug’s safety over the long term.
Rajesh Gandhi, an infectious diseases physician at Massachusetts General Hospital and Harvard Medical School, said he would like to know if the pill actually shortened the duration of symptoms and decreased the severity of the disease for those patients who were hospitalized.
He said if the drug is authorized and when more data become available, he would likely prescribe the drug to outpatients with a risk factor, in line with the group that Merck and Ridgeback studied, rather than those at low risk, and that antivirals are best started early in the course of a disease. Yet the drug, he said, would be helpful for those at risk of severe illness.
“You could call in a prescription for the patient, they wouldn’t have to come to get an infusion or a subcutaneous injection, and therefore it would make it easier, kind of like how we treat influenza during flu season,” he said.
Molnupiravir was initially developed by a not-for-profit biotechnology company owned by Emory University. Ridgeback then licensed and formed a collaboration with Emory in early 2020 and later announced a partnership with Merck.
Molnupiravir works by attacking a different portion of the virus than the spike protein on the coronavirus commonly targeted by Covid-19 vaccines and other Covid-19 drugs. The portion attacked by molnupiravir helps the virus reproduce.
“You will now have an oral, easily accessible, easily distributed medicine to keep people out of the hospital and to keep them from dying,” said Wayne Holman, co-founder of Miami-based Ridgeback.
Other companies are also developing Covid-19 antivirals, including Pfizer Inc., as well as Roche Holding AG and Atea Pharmaceuticals Inc.
Merck and Ridgeback stopped a study of molnupiravir in hospitalized patients in April, after researchers found that it failed to help them and is unlikely to reduce hospital stays and deaths.
The companies continued studying whether the antiviral was effective early in the course of the disease and in people who are at high risk of Covid-19 complications. Antivirals tend to be most effective when taken soon after infection and become less beneficial over time as patients get sicker, according to doctors and scientists.
The Merck-Ridgeback late-stage, or Phase 3, study enrolled more than 1,400 people who were at high risk of becoming seriously ill with Covid-19. To enroll, participants had to be unvaccinated. About half received an 800-milligram dose of molnupiravir twice a day for five days, with the other participants receiving a placebo.
High risk was defined as having at least one characteristic associated with severe disease or death, such as old age, obesity or diabetes. Treatment began within five days after the participants developed Covid-19 symptoms.
In the interim analysis, 28 of the 385 subjects who received the drug were hospitalized after 29 days, Merck and Ridgeback said, compared with 53 of the 377 subjects in the placebo group who were either hospitalized or died, resulting in an efficacy rate of about 50%.
Through the 29 days, no subjects who received molnupiravir had died, compared with eight deaths in the placebo group, according to the companies.
The interim review of the drug’s effectiveness and safety was performed by an outside panel of independent experts known as a data-safety monitoring committee, which then shared its findings with Merck and Ridgeback.
The panel met late Tuesday to review the data, and then recommended the study stop enrollment because of the positive results, Dr. Holman said. The companies then went to the FDA with that recommendation.
Mr. Davis said that researchers would still perform a final analysis, which could mean the efficacy percentage is adjusted but the overall positive outcome shouldn’t change.
Merck and Ridgeback said in September that they had begun a separate trial studying whether molnupiravir could prevent infection in people after they are exposed to the virus.
Updated: 10-5-2021
Everything You Need to Know About Merck’s Game-Changing Covid Pill
Molnupiravir, an antiviral pill being developed by Merck & Co., has been touted as a potential game changer in the fight against Covid-19. The experimental medication was shown to reduce the risk of hospitalization or death by about half in a late-stage study of adults with mild-to-moderate cases. The promise of a drug that patients can easily get and take at home has prompted some governments to order supplies even before regulators have decided whether to approve its use.
1. What Is Molnupiravir?
It’s the chemical name for a medicine originally developed to treat influenza that’s given orally in a capsule. It inhibits replication of SARS-CoV-2, the coronavirus that causes Covid, by a mechanism known as “lethal mutagenesis.”
In simple terms, it causes the machinery that reproduces the virus’s genetic material to make mistakes, thereby rendering the copies defective. The drug was discovered at Emory University in Atlanta, and is being developed by Kenilworth, New Jersey-based Merck and Miami-based Ridgeback Biotherapeutics LP.
2. How Effective Is It?
Interim analysis of data from a randomized trial found that it cut the risk of hospitalization by about 50%, Merck said in an Oct. 1 statement. Of 385 patients who got the drug, 28 or 7.3% were hospitalized, compared with 53 out of 377 (14.1%) who got a placebo. Through day 29, no deaths were reported in patients who received molnupiravir, but eight died in the placebo arm. The study was relatively small, and further research is required.
But results were so encouraging that Merck and Ridgeback, in consultation with independent trial monitors and the U.S. Food and Drug Administration, halted the trial and began the process of gaining regulatory clearance. The company said at a conference in September that early research showed molnupiravir can thwart the most common SARS-CoV-2 variants, including delta and gamma.
3. How Is It Different From Other Drugs?
Gilead Sciences Inc.’s antiviral remdesivir, as well as monoclonal antibodies, are administered via an intravenous infusion. This is usually done in a hospital or a clinic, where infected people risk transmitting the virus to medical staff and other patients. Molnupiravir’s main advantage is that it’s taken as a pill, enabling patients to be treated at home.
It’s also likely to be cheaper: A five-day course of molnupiravir will cost about $700 per patient — a third of the cost of a monoclonal antibody treatment, according to the New York Times. Safe, well-tolerated, affordable and easy-to-administer antivirals are ideal treatments because they directly counter the virus, limiting its damage to the body and the duration of illness. Steroids and blood-thinners that have been shown to improve survival in hospitalized patients don’t directly fight the virus; rather they prevent a worsening of Covid symptoms.
4. How Was It Administered?
Molnupiravir was taken orally every 12 hours for five days by adults with mild-to-moderate Covid. Studies are still underway to determine the most efficacious regimen. A study earlier this year showed molnupiravir had little effect when it was given to patients already hospitalized with severe disease. One study is testing whether it can be used to prevent SARS-CoV-2 spreading in households in which one or more members have Covid.
5. Are There Side Effects?
Interim analysis found no increased incidence of adverse events. Only 1.3% of participants taking molnupiravir quit the therapy due to an adverse event, compared with 3.4% in the placebo group. Still, molnupiravir will need to be assessed in a much larger group of patients to properly determine its safety. People involved in the trial were instructed to abstain from heterosexual sex or use contraception. While this is routine practice with some other medicines, such as cancer chemotherapy, it suggests that molnupiravir has the potential to cause birth defects should someone become pregnant.
6. What About The Supply Of Molnupiravir?
Merck expects to make 10 million treatment courses by the end of 2021, with more doses slated for production in 2022. The drugmaker agreed in June to a $1.2 billion supply deal with the U.S. government under which it would provide 1.7 million courses of the treatment once the drug gains emergency use authorization or approval from the FDA. Merck also has deals with other governments, pending regulatory authorization.
For instance, Australia ordered 300,000 doses. Merck says it will implement a tiered-pricing approach based on World Bank country income criteria to reflect countries’ relative ability to finance their health response to the pandemic. The drugmaker announced in April voluntary licensing agreements with generic-drug manufacturers in India to accelerate molnupiravir’s availability in more than 100 low- and middle-income countries as soon as the medication gains the necessary approvals.
7. Will It Replace The Need For Vaccines?
No. Vaccination remains the most effective shield against Covid-19. Still, Covid-19 cases are continuing to occur even in highly vaccinated populations, meaning antiviral therapies will play an important role in limiting the disease’s severity, especially in the elderly and people with weakened immune systems for whom vaccination is less effective. The best first line of defense against this disease is to not get infected in the first place. Social and public health measures, such as wearing face masks and maintaining a physical distance, are still key to preventing virus transmission and ending Covid epidemics.
Highly convenient oral drugs could be a pandemic game changer. With enough investment, the U.S. could make a whole lot of them for the world.
President Joe Biden unveiled big plans earlier this month for the U.S. fight against Covid-19, anchored by expansive vaccine mandates and a planned rollout of booster shots. On Wednesday, he turned his focus abroad, hosting a virtual vaccine summit with global leaders aimed at protecting the world. But there’s more he can do.
Three pharmaceutical giants — Merck & Co., Pfizer Inc. and Roche Holding AG — are in the late stages of testing oral antiviral drugs (Merck in partnership with Ridgeback Biotherapeutics LP and Roche with Atea Pharmaceuticals Inc.) that aim to block the Covid-19 virus from replicating.
If given early in the course of infection, these medicines have the potential to keep people from getting severely ill or ending up in the hospital. Because pills are relatively cheap to manufacture in large amounts and easy to distribute, expanded funding could make them a pandemic game changer.
The U.S. has already promised to buy a limited supply of Merck’s treatment and announced $3.2 billion in general antiviral investment in June. But investing more in all three programs ahead of authorization could ensure ample supply of what may be very useful medicines to deploy as soon as regulators give the go-ahead.
It’s a strategy the country used earlier in the pandemic under the Trump administration, with deals that gave companies cash up front to build up inventories of vaccines and antibodies. With these new oral treatments, Biden can follow the same playbook, only at a larger scale and with faster results. Data is due from key clinical trials of all three leading candidates in the next few months, and while failure could see money wasted, the potential rewards are worth the risk.
Compared to antibodies and vaccines, which are biologic products that are expensive to manufacture and can have burdensome storage or delivery requirements, these antivirals are refreshingly straightforward. Pills can be stamped out in large volumes using widely available equipment and dispensed through local pharmacies or clinics with no needles or freezers required.
A course of Merck’s pill will cost the U.S. a third of what it has paid for Regeneron Pharmaceuticals Inc.’s antibody therapy, if it proves successful. That’s even before accounting for the much higher cost of administering antibodies.
On the subject of antibodies, supplies remain inadequate in the U.S. and absent in many countries. As for vaccinations, as of Sept. 9, only 2% of people in low-income countries had received one dose. Fixing this is crucial, but supply chains are stretched, and additional capacity will take time to come on line. In that context, it’s crucial to also leverage the potential advantages of oral treatments.
The continuing popularity of ivermectin, a drug for parasitic diseases that hasn’t shown firm evidence of efficacy, makes it clear that there’s substantial demand for a convenient option that really works.
The relative ease, speed, and economy of pill production could result — with sufficient investment and trial success — in enough timely doses for the entire world. The biggest benefit of expansive supply would come in low-income nations otherwise left behind. Millions will remain vulnerable for months to come as they wait for vaccines, making treatments a crucial protective bridge.
And the value of an easily dispensed treatment that keeps people from getting severely ill is higher in places with weaker infrastructure. The key is to spend early, be ambitious, and focus on direct financing of global manufacturing capacity.
“This is a global tragedy,” Biden said at the vaccine summit. “And we’re not going to solve this crisis with half-measures and middle-of-the-road ambitions. We need to go big and do our part.”
He’s right. But he needs to think beyond the shot.
Related Articles:
Bitcoin Information & Resources (#GotBitcoin)
Ultimate Resource For Nationwide Firsts Taking Place In California (#GotBitcoin)
Flight To Money Funds Is Adding To The Strains On Banks #GotBitcoin
The Fed Loses Money For The First Time In 107 Years – Why It Matters #GotBitcoin
African Safari Vacation Itinerary (2024 Proposal)
The Next Fountain-of-Youth Craze? Peptide Injections
This Ocean Monster Offers A Potential Climate Solution
What Are Credit Default Swaps, How Do They Work, And How They Go Wrong
Cyberattack Sends Quadrillion Dollars Derivative’s Trading Markets Back To The 1980s #GotBitcoin
What Is Dopamine Fasting? Meet The Dangerous Fad Among Silicon Valley’s Tech Geniuses
Bitcoin Community Leaders Join Longevity Movement
Sean Harribance Shares His Psychic Gifts With The Public
Scientists Achieve Real-Time Communication With Lucid Dreamers In Breakthrough
Does Getting Stoned Help You Get Toned? Gym Rats Embrace Marijuana
Marijuana In Africa Is Like The Gold Rush For America In The 1800s
The Perfect Wine And Weed To Get You Through The Coronavirus Pandemic Lockdown
Mike Tyson’s 420-Acre Weed Ranch Rakes In $500K A Month
What Sex Workers Want To Do With Bitcoin
“Is Bitcoin Reacting To The Chaos Or Is Bitcoin Causing The Chaos?” Max Keiser
How To Safely Store Deposits If You Have More Than $250,000
How To Host A Decentralized Website
Banks Lose Billions (Approx. $52 Billion) As Depositors Seek Higher Deposit Yields #GotBitcoin
Crypto User Recovers Long-Lost Private Keys To Access $4M In Bitcoin
Stripe Stops Processing Payments For Trump Campaign Website
Bitcoin Whales Are Profiting As ‘Weak Hands’ Sell BTC After Price Correction
Pentagon Sees Giant Cargo Cranes As Possible Chinese Spying Tools
Bitcoin’s Volatility Should Burn Investors. It Hasn’t
Bitcoin’s Latest Record Run Is Less Volatile Than The 2017 Boom
“Lettuce Hands” Refers To Investors Who Can’t Deal With The Volatility Of The Cryptocurrency Markets
The Bitcoiners Who Live Off The Grid
US Company Now Lets Travelers Pay For Passports With Bitcoin
After A Year Without Rowdy Tourists, European Cities Want To Keep It That Way
Director Barry Jenkins Is The Travel Nerd’s Travel Nerd
Four Stories Of How People Traveled During Covid
Who Is A Perpetual Traveler (AKA Digital Nomad) Under The US Tax Code
Tricks For Making A Vacation Feel Longer—And More Fulfilling
Travel Has Bounced Back From Coronavirus, But Tourists Stick Close To Home
Nurses Travel From Coronavirus Hot Spot To Hot Spot, From New York To Texas
How To Travel Luxuriously Post- Covid-19, From Private Jets To Hotel Buyouts
Ultimate Travel Resource Covering Business, Personal, Cruise, Flying, Etc.)
Does Bitcoin Boom Mean ‘Better Gold’ Or Bigger Bubble?
Bitcoin’s Slide Dents Price Momentum That Dwarfed Everything
Retail Has Arrived As Paypal Clears $242M In Crypto Sales Nearly Double The Previous Record
Jarlsberg Cheese Offers Significant Bone & Heart-Health Benefits Thanks To Vitamin K2, Says Study
Chrono-Pharmacology Reveals That “When” You Take Your Medication Can Make A Life-Saving Difference
Ultimate Resource For News, Breakthroughs And Innovations In Healthcare
Ultimate Resource For Cooks, Chefs And The Latest Food Trends
Ethereum Use Cases You Might Not Know
Will 1% Yield Force The Fed Into Curve Control?
Ultimate Resource On Hong Kong Vying For World’s Crypto Hub #GotBitcoin
France Moves To Ban Anonymous Crypto Accounts To Prevent Money Laundering
Numerous Times That US (And Other) Regulators Stepped Into Crypto
Where Does This 28% Bitcoin Price Drop Rank In History? Not Even In The Top 5
Traditional Investors View Bitcoin As If It Were A Technology Stock
3 Reasons Why Bitcoin Price Abruptly Dropped 6% After Reaching $15,800
As Bitcoin Approaches $25,000 It Breaks Correlation With Equities
UK Treasury Calls For Feedback On Approach To Cryptocurrency And Stablecoin Regulation
Bitcoin Rebounds While Leaving Everyone In Dark On True Worth
Slow-Twitch vs. Fast-Twitch Muscle Fibers
Biden, Obama Release Campaign Video Applauding Their Achievements
Joe Biden Tops Donald Trump In Polls And Leads In Fundraising (#GotBitcoin)
Trump Gets KPOP’d And Tic Toc’d As Teens Mobilized To Derail Trump’s Tulsa Rally
Schwab’s $200 Million Charge Puts Scrutiny On Robo-Advising
TikTok Is The Place To Go For Financial Advice If You’re A Young Adult
TikTok Is The Place To Go For Financial Advice If You’re A Young Adult
American Shoppers Just Can’t Pass Up A Bargain And Department Stores Pay The Price #GotBitcoin
Motley Fool Adding $5M In Bitcoin To Its ‘10X Portfolio’ — Has A $500K Price Target
Mad Money’s Jim Cramer Invests 1% Of Net Worth In Bitcoin Says, “Gold Is Dangerous”
Ultimate Resource For Financial Advisers By Financial Advisers On Crypto
Anti-ESG Movement Reveals How Blackrock Pulls-off World’s Largest Ponzi Scheme
The Bitcoin Ordinals Protocol Has Caused A Resurgence In Bitcoin Development And Interest
Bitcoin Takes ‘Lion’s Share’ As Institutional Inflows Hit 7-Month High
Bitcoin’s Future Depends On A Handful of Mysterious Coders
Billionaire Hedge Fund Investor Stanley Druckenmiller Says He Owns Bitcoin In CNBC Interview
Bitcoin Billionaire Chamath Palihapitiya Opts Out Of Run For California Governor
Billionaire Took Psychedelics, Got Bitcoin And Is Now Into SPACs
Billionaire’s Bitcoin Dream Shapes His Business Empire In Norway
Trading Firm Of Richest Crypto Billionaire Reveals Buying ‘A Lot More’ Bitcoin Below $30K
Simple Tips To Ensure Your Digital Surveillance Works As It Should
Big (4) Audit Firms Blasted By PCAOB And Gary Gensler, Head Of SEC (#GotBitcoin)
What Crypto Users Need Know About Changes At The SEC
The Ultimate Resource For The Bitcoin Miner And The Mining Industry (Page#2) #GotBitcoin
How Cryptocurrency Can Help In Paying Universal Basic Income (#GotBitcoin)
Gautam Adani Was Briefly World’s Richest Man Only To Be Brought Down By An American Short-Seller
Global Crypto Industry Pledges Aid To Turkey Following Deadly Earthquakes
Money Supply Growth Went Negative Again In December Another Sign Of Recession #GotBitcoin
Here Is How To Tell The Difference Between Bitcoin And Ethereum
Crypto Investors Can Purchase Bankruptcy ‘Put Options’ To Protect Funds On Binance, Coinbase, Kraken
Bitcoin Developers Must Face UK Trial Over Lost Cryptoassets
Google Issues Warning For 2 Billion Chrome Users
How A Lawsuit Against The IRS Is Trying To Expand Privacy For Crypto Users
IRS Uses Cellphone Location Data To Find Suspects
IRS Failed To Collect $2.4 Billion In Taxes From Millionaires
Treasury Calls For Crypto Transfers Over $10,000 To Be Reported To IRS
Can The IRS Be Trusted With Your Data?
US Ransomware Attack Suspect Hails From A Small Ukrainian Town
Alibaba Admits It Was Slow To Report Software Bug After Beijing Rebuke
Japan Defense Ministry Finds Security Threat In Hack
Raoul Pal Believes Institutions Have Finished Taking Profits As Year Winds Up
Yosemite Is Forcing Native American Homeowners To Leave Without Compensation. Here’s Why
What Is Dollar Cost Averaging Bitcoin?
Ultimate Resource On Bitcoin Unit Bias
Best Travel Credit Cards of 2022-2023
A Guarded Generation: How Millennials View Money And Investing (#GotBitcoin)
Bitcoin Enthusiast And CEO Brian Armstrong Buys Los Angeles Home For $133 Million
Nasdaq-Listed Blockchain Firm BTCS To Offer Dividend In Bitcoin; Shares Surge
Ultimate Resource On Kazakhstan As Second In Bitcoin Mining Hash Rate In The World After US
Ultimate Resource On Solana Outages And DDoS Attacks
How Jessica Simpson Almost Lost Her Name And Her Billion Dollar Empire
Sidney Poitier, Actor Who Made Oscars History, Dies At 94
Green Comet Will Be Visible As It Passes By Earth For First Time In 50,000 Years
FTX (SBF) Got Approval From F.D.I.C., State Regulators And Federal Reserve To Buy Tiny Bank!!!
Joe Rogan: I Have A Lot Of Hope For Bitcoin
Teen Cyber Prodigy Stumbled Onto Flaw Letting Him Hijack Teslas
Spyware Finally Got Scary Enough To Freak Lawmakers Out—After It Spied On Them
The First Nuclear-Powered Bitcoin Mine Is Here. Maybe It Can Clean Up Energy FUD
The World’s Best Crypto Policies: How They Do It In 37 Nations
Tonga To Copy El Salvador’s Bill Making Bitcoin Legal Tender, Says Former MP
Wordle Is The New “Lingo” Turning Fans Into Argumentative Strategy Nerds
Prospering In The Pandemic, Some Feel Financial Guilt And Gratitude
Is Art Therapy The Path To Mental Well-Being?
New York, California, New Jersey, And Alabama Move To Ban ‘Forever Chemicals’ In Firefighting Foam
The Mystery Of The Wasting House-Cats
What Pet Owners Should Know About Chronic Kidney Disease In Dogs And Cats
Pets Score Company Perks As The ‘New Dependents’
Why Is My Cat Rubbing His Face In Ants?
Natural Cure For Hyperthyroidism In Cats Including How To Switch Him/Her To A Raw Food Diet
Ultimate Resource For Cat Lovers
FDA Approves First-Ever Arthritis Pain Management Drug For Cats
Ultimate Resource On Duke of York’s Prince Andrew And His Sex Scandal
Walmart Filings Reveal Plans To Create Cryptocurrency, NFTs
Bitcoin’s Dominance of Crypto Payments Is Starting To Erode
T-Mobile Says Hackers Stole Data On About 37 Million Customers
Jack Dorsey Announces Bitcoin Legal Defense Fund
More Than 100 Millionaires Signed An Open Letter Asking To Be Taxed More Heavily
Federal Regulator Says Credit Unions Can Partner With Crypto Providers
What’s Behind The Fascination With Smash-And-Grab Shoplifting?
Train Robberies Are A Problem In Los Angeles, And No One Agrees On How To Stop Them
US Stocks Historically Deliver Strong Gains In Fed Hike Cycles (GotBitcoin)
Ian Alexander Jr., Only Child of Regina King, Dies At Age 26
Amazon Ends Its Charity Donation Program Amazonsmile After Other Cost-Cutting Efforts
Indexing Is Coming To Crypto Funds Via Decentralized Exchanges
Doctors Show Implicit Bias Towards Black Patients
Darkmail Pushes Privacy Into The Hands Of NSA-Weary Customers
3D Printing Make Anything From Candy Bars To Hand Guns
Stealing The Blood Of The Young May Make You More Youthful
Henrietta Lacks And Her Remarkable Cells Will Finally See Some Payback
AL_A Wins Approval For World’s First Magnetized Fusion Power Plant
Want To Be Rich? Bitcoin’s Limited Supply Cap Means You Only Need 0.01 BTC
Smart Money Is Buying Bitcoin Dip. Stocks, Not So Much
McDonald’s Jumps On Bitcoin Memewagon, Crypto Twitter Responds
America COMPETES Act Would Be Disastrous For Bitcoin Cryptocurrency And More
Lyn Alden On Bitcoin, Inflation And The Potential Coming Energy Shock
Inflation And A Tale of Cantillionaires
El Salvador Plans Bill To Adopt Bitcoin As Legal Tender
Miami Mayor Says City Employees Should Be Able To Take Their Salaries In Bitcoin
Vast Troves of Classified Info Undermine National Security, Spy Chief Says
BREAKING: Arizona State Senator Introduces Bill To Make Bitcoin Legal Tender
San Francisco’s Historic Surveillance Law May Get Watered Down
How Bitcoin Contributions Funded A $1.4M Solar Installation In Zimbabwe
California Lawmaker Says National Privacy Law Is a Priority
The Pandemic Turbocharged Online Privacy Concerns
How To Protect Your Online Privacy While Working From Home
Researchers Use GPU Fingerprinting To Track Users Online
Japan’s $1 Trillion Crypto Market May Ease Onerous Listing Rules
Ultimate Resource On A Weak / Strong Dollar’s Impact On Bitcoin
Fed Money Printer Goes Into Reverse (Quantitative Tightening): What Does It Mean For Crypto?
Crypto Market Is Closer To A Bottom Than Stocks (#GotBitcoin)
When World’s Central Banks Get It Wrong, Guess Who Pays The Price😂😹🤣 (#GotBitcoin)
“Better Days Ahead With Crypto Deleveraging Coming To An End” — Joker
Crypto Funds Have Seen Record Investment Inflow In Recent Weeks
Bitcoin’s Epic Run Is Winning More Attention On Wall Street
Ultimate Resource For Crypto Mergers And Acquisitions (M&A) (#GotBitcoin)
Why Wall Street Is Literally Salivating Over Bitcoin
Nasdaq-Listed MicroStrategy And Others Wary Of Looming Dollar Inflation, Turns To Bitcoin And Gold
Bitcoin For Corporations | Michael Saylor | Bitcoin Corporate Strategy
Ultimate Resource On Myanmar’s Involvement With Crypto-Currencies
‘I Cry Every Day’: Olympic Athletes Slam Food, COVID Tests And Conditions In Beijing
Does Your Baby’s Food Contain Toxic Metals? Here’s What Our Investigation Found
Ultimate Resource For Pro-Crypto Lobbying And Non-Profit Organizations
Ultimate Resource On BlockFi, Celsius And Nexo
Petition Calling For Resignation Of U.S. Securities/Exchange Commission Chair Gary Gensler
100 Million Americans Can Legally Bet on the Super Bowl. A Spot Bitcoin ETF? Forget About it!
Green Finance Isn’t Going Where It’s Needed
Shedding Some Light On The Murky World Of ESG Metrics
SEC Targets Greenwashers To Bring Law And Order To ESG
BlackRock (Assets Under Management $7.4 Trillion) CEO: Bitcoin Has Caught Our Attention
Canada’s Major Banks Go Offline In Mysterious (Bank Run?) Hours-Long Outage (#GotBitcoin)
On-Chain Data: A Framework To Evaluate Bitcoin
On Its 14th Birthday, Bitcoin’s 1,690,706,971% Gain Looks Kind of… Well Insane
The Most Important Health Metric Is Now At Your Fingertips
American Bargain Hunters Flock To A New Online Platform Forged In China
Why We Should Welcome Another Crypto Winter
Traders Prefer Gold, Fiat Safe Havens Over Bitcoin As Russia Goes To War
Music Distributor DistroKid Raises Money At $1.3 Billion Valuation
Nas Selling Rights To Two Songs Via Crypto Music Startup Royal
Ultimate Resource On Music Catalog Deals
Ultimate Resource On Music And NFTs And The Implications For The Entertainment Industry
Lead And Cadmium Could Be In Your Dark Chocolate
Catawba, Native-American Tribe Approves First Digital Economic Zone In The United States
The Miracle Of Blockchain’s Triple Entry Accounting
How And Why To Stimulate Your Vagus Nerve!
Housing Boom Brings A Shortage Of Land To Build New Homes
Biden Lays Out His Blueprint For Fair Housing
No Grave Dancing For Sam Zell Now. He’s Paying Up For Hot Properties
Cracks In The Housing Market Are Starting To Show
Ever-Growing Needs Strain U.S. Food Bank Operations
Food Pantry Helps Columbia Students Struggling To Pay Bills
Food Insecurity Driven By Climate Change Has Central Americans Fleeing To The U.S.
Housing Insecurity Is Now A Concern In Addition To Food Insecurity
Families Face Massive Food Insecurity Levels
US Troops Going Hungry (Food Insecurity) Is A National Disgrace
Everything You Should Know About Community Fridges, From Volunteering To Starting Your Own
Russia’s Independent Journalists Including Those Who Revealed The Pandora Papers Need Your Help
10 Women Who Used Crypto To Make A Difference In 2021
Happy International Women’s Day! Leaders Share Their Experiences In Crypto
Dollar On Course For Worst Performance In Over A Decade (#GotBitcoin)
Juice The Stock Market And Destroy The Dollar!! (#GotBitcoin)
Unusual Side Hustles You May Not Have Thought Of
Ultimate Resource On Global Inflation And Rising Interest Rates (#GotBitcoin)
The Fed Is Setting The Stage For Hyper-Inflation Of The Dollar (#GotBitcoin)
An Antidote To Inflation? ‘Buy Nothing’ Groups Gain Popularity
Why Is Bitcoin Dropping If It’s An ‘Inflation Hedge’?
Lyn Alden Talks Bitcoin, Inflation And The Potential Coming Energy Shock
Ultimate Resource On How Black Families Can Fight Against Rising Inflation (#GotBitcoin)
What The Fed’s Rate Hike Means For Inflation, Housing, Crypto And Stocks
Egyptians Buy Bitcoin Despite Prohibitive New Banking Laws
Archaeologists Uncover Five Tombs In Egypt’s Saqqara Necropolis
History of Alchemy From Ancient Egypt To Modern Times
Former World Bank Chief Didn’t Act On Warnings Of Sexual Harassment
Does Your Hospital or Doctor Have A Financial Relationship With Big Pharma?
Ultimate Resource Covering The Crisis Taking Place In The Nickel Market
Apple Along With Meta And Secret Service Agents Fooled By Law Enforcement Impersonators
Handy Tech That Can Support Your Fitness Goals
How To Naturally Increase Your White Blood Cell Count
Ultimate Source For Russians Oligarchs And The Impact Of Sanctions On Them
Ultimate Source For Bitcoin Price Manipulation By Wall Street
Russia, Sri Lanka And Lebanon’s Defaults Could Be The First Of Many (#GotBitcoin)
Will Community Group Buying Work In The US?
Building And Running Businesses In The ‘Spirit Of Bitcoin’
What Is The Mysterious Liver Disease Hurting (And Killing) Children?
Citigroup Trader Is Scapegoat For Flash Crash In European Stocks (#GotBitcoin)
Bird Flu Outbreak Approaches Worst Ever In U.S. With 37 Million Animals Dead
Financial Inequality Grouped By Race For Blacks, Whites And Hispanics
How Black Businesses Can Prosper From Targeting A Trillion-Dollar Black Culture Market (#GotBitcoin)
Ultimate Resource For Central Bank Digital Currencies (#GotBitcoin) Page#2
Meet The Crypto Angel Investor Running For Congress In Nevada (#GotBitcoin?)
Introducing BTCPay Vault – Use Any Hardware Wallet With BTCPay And Its Full Node (#GotBitcoin?)
How Not To Lose Your Coins In 2020: Alternative Recovery Methods (#GotBitcoin?)
H.R.5635 – Virtual Currency Tax Fairness Act of 2020 ($200.00 Limit) 116th Congress (2019-2020)
Adam Back On Satoshi Emails, Privacy Concerns And Bitcoin’s Early Days
The Prospect of Using Bitcoin To Build A New International Monetary System Is Getting Real
How To Raise Funds For Australia Wildfire Relief Efforts (Using Bitcoin And/Or Fiat )
Former Regulator Known As ‘Crypto Dad’ To Launch Digital-Dollar Think Tank (#GotBitcoin?)
Currency ‘Cold War’ Takes Center Stage At Pre-Davos Crypto Confab (#GotBitcoin?)
A Blockchain-Secured Home Security Camera Won Innovation Awards At CES 2020 Las Vegas
Bitcoin’s Had A Sensational 11 Years (#GotBitcoin?)
Sergey Nazarov And The Creation Of A Decentralized Network Of Oracles
Google Suspends MetaMask From Its Play App Store, Citing “Deceptive Services”
Christmas Shopping: Where To Buy With Crypto This Festive Season
At 8,990,000% Gains, Bitcoin Dwarfs All Other Investments This Decade
Coinbase CEO Armstrong Wins Patent For Tech Allowing Users To Email Bitcoin
Bitcoin Has Got Society To Think About The Nature Of Money
How DeFi Goes Mainstream In 2020: Focus On Usability (#GotBitcoin?)
Dissidents And Activists Have A Lot To Gain From Bitcoin, If Only They Knew It (#GotBitcoin?)
At A Refugee Camp In Iraq, A 16-Year-Old Syrian Is Teaching Crypto Basics
Bitclub Scheme Busted In The US, Promising High Returns From Mining
Bitcoin Advertised On French National TV
Germany: New Proposed Law Would Legalize Banks Holding Bitcoin
How To Earn And Spend Bitcoin On Black Friday 2019
The Ultimate List of Bitcoin Developments And Accomplishments
Charities Put A Bitcoin Twist On Giving Tuesday
Family Offices Finally Accept The Benefits of Investing In Bitcoin
An Army Of Bitcoin Devs Is Battle-Testing Upgrades To Privacy And Scaling
Bitcoin ‘Carry Trade’ Can Net Annual Gains With Little Risk, Says PlanB
Max Keiser: Bitcoin’s ‘Self-Settlement’ Is A Revolution Against Dollar
Blockchain Can And Will Replace The IRS
China Seizes The Blockchain Opportunity. How Should The US Respond? (#GotBitcoin?)
Jack Dorsey: You Can Buy A Fraction Of Berkshire Stock Or ‘Stack Sats’
Bitcoin Price Skyrockets $500 In Minutes As Bakkt BTC Contracts Hit Highs
Bitcoin’s Irreversibility Challenges International Private Law: Legal Scholar
Bitcoin Has Already Reached 40% Of Average Fiat Currency Lifespan
Yes, Even Bitcoin HODLers Can Lose Money In The Long-Term: Here’s How (#GotBitcoin?)
Unicef To Accept Donations In Bitcoin (#GotBitcoin?)
Former Prosecutor Asked To “Shut Down Bitcoin” And Is Now Face Of Crypto VC Investing (#GotBitcoin?)
Switzerland’s ‘Crypto Valley’ Is Bringing Blockchain To Zurich
Next Bitcoin Halving May Not Lead To Bull Market, Says Bitmain CEO
Bitcoin Developer Amir Taaki, “We Can Crash National Economies” (#GotBitcoin?)
Veteran Crypto And Stocks Trader Shares 6 Ways To Invest And Get Rich
Is Chainlink Blazing A Trail Independent Of Bitcoin?
Nearly $10 Billion In BTC Is Held In Wallets Of 8 Crypto Exchanges (#GotBitcoin?)
SEC Enters Settlement Talks With Alleged Fraudulent Firm Veritaseum (#GotBitcoin?)
Blockstream’s Samson Mow: Bitcoin’s Block Size Already ‘Too Big’
Attorneys Seek Bank Of Ireland Execs’ Testimony Against OneCoin Scammer (#GotBitcoin?)
OpenLibra Plans To Launch Permissionless Fork Of Facebook’s Stablecoin (#GotBitcoin?)
Tiny $217 Options Trade On Bitcoin Blockchain Could Be Wall Street’s Death Knell (#GotBitcoin?)
Class Action Accuses Tether And Bitfinex Of Market Manipulation (#GotBitcoin?)
Sharia Goldbugs: How ISIS Created A Currency For World Domination (#GotBitcoin?)
Bitcoin Eyes Demand As Hong Kong Protestors Announce Bank Run (#GotBitcoin?)
How To Securely Transfer Crypto To Your Heirs
‘Gold-Backed’ Crypto Token Promoter Karatbars Investigated By Florida Regulators (#GotBitcoin?)
Crypto News From The Spanish-Speaking World (#GotBitcoin?)
Financial Services Giant Morningstar To Offer Ratings For Crypto Assets (#GotBitcoin?)
‘Gold-Backed’ Crypto Token Promoter Karatbars Investigated By Florida Regulators (#GotBitcoin?)
The Original Sins Of Cryptocurrencies (#GotBitcoin?)
Bitcoin Is The Fraud? JPMorgan Metals Desk Fixed Gold Prices For Years (#GotBitcoin?)
Israeli Startup That Allows Offline Crypto Transactions Secures $4M (#GotBitcoin?)
[PSA] Non-genuine Trezor One Devices Spotted (#GotBitcoin?)
Bitcoin Stronger Than Ever But No One Seems To Care: Google Trends (#GotBitcoin?)
First-Ever SEC-Qualified Token Offering In US Raises $23 Million (#GotBitcoin?)
You Can Now Prove A Whole Blockchain With One Math Problem – Really
Crypto Mining Supply Fails To Meet Market Demand In Q2: TokenInsight
$2 Billion Lost In Mt. Gox Bitcoin Hack Can Be Recovered, Lawyer Claims (#GotBitcoin?)
Fed Chair Says Agency Monitoring Crypto But Not Developing Its Own (#GotBitcoin?)
Wesley Snipes Is Launching A Tokenized $25 Million Movie Fund (#GotBitcoin?)
Mystery 94K BTC Transaction Becomes Richest Non-Exchange Address (#GotBitcoin?)
A Crypto Fix For A Broken International Monetary System (#GotBitcoin?)
Four Out Of Five Top Bitcoin QR Code Generators Are Scams: Report (#GotBitcoin?)
Waves Platform And The Abyss To Jointly Launch Blockchain-Based Games Marketplace (#GotBitcoin?)
Bitmain Ramps Up Power And Efficiency With New Bitcoin Mining Machine (#GotBitcoin?)
Ledger Live Now Supports Over 1,250 Ethereum-Based ERC-20 Tokens (#GotBitcoin?)
Miss Finland: Bitcoin’s Risk Keeps Most Women Away From Cryptocurrency (#GotBitcoin?)
Artist Akon Loves BTC And Says, “It’s Controlled By The People” (#GotBitcoin?)
Ledger Live Now Supports Over 1,250 Ethereum-Based ERC-20 Tokens (#GotBitcoin?)
Co-Founder Of LinkedIn Presents Crypto Rap Video: Hamilton Vs. Satoshi (#GotBitcoin?)
Crypto Insurance Market To Grow, Lloyd’s Of London And Aon To Lead (#GotBitcoin?)
No ‘AltSeason’ Until Bitcoin Breaks $20K, Says Hedge Fund Manager (#GotBitcoin?)
NSA Working To Develop Quantum-Resistant Cryptocurrency: Report (#GotBitcoin?)
Custody Provider Legacy Trust Launches Crypto Pension Plan (#GotBitcoin?)
Vaneck, SolidX To Offer Limited Bitcoin ETF For Institutions Via Exemption (#GotBitcoin?)
Russell Okung: From NFL Superstar To Bitcoin Educator In 2 Years (#GotBitcoin?)
Bitcoin Miners Made $14 Billion To Date Securing The Network (#GotBitcoin?)
Why Does Amazon Want To Hire Blockchain Experts For Its Ads Division?
Argentina’s Economy Is In A Technical Default (#GotBitcoin?)
Blockchain-Based Fractional Ownership Used To Sell High-End Art (#GotBitcoin?)
Portugal Tax Authority: Bitcoin Trading And Payments Are Tax-Free (#GotBitcoin?)
Bitcoin ‘Failed Safe Haven Test’ After 7% Drop, Peter Schiff Gloats (#GotBitcoin?)
Bitcoin Dev Reveals Multisig UI Teaser For Hardware Wallets, Full Nodes (#GotBitcoin?)
Bitcoin Price: $10K Holds For Now As 50% Of CME Futures Set To Expire (#GotBitcoin?)
Bitcoin Realized Market Cap Hits $100 Billion For The First Time (#GotBitcoin?)
Stablecoins Begin To Look Beyond The Dollar (#GotBitcoin?)
Bank Of England Governor: Libra-Like Currency Could Replace US Dollar (#GotBitcoin?)
Binance Reveals ‘Venus’ — Its Own Project To Rival Facebook’s Libra (#GotBitcoin?)
The Real Benefits Of Blockchain Are Here. They’re Being Ignored (#GotBitcoin?)
CommBank Develops Blockchain Market To Boost Biodiversity (#GotBitcoin?)
SEC Approves Blockchain Tech Startup Securitize To Record Stock Transfers (#GotBitcoin?)
SegWit Creator Introduces New Language For Bitcoin Smart Contracts (#GotBitcoin?)
You Can Now Earn Bitcoin Rewards For Postmates Purchases (#GotBitcoin?)
Bitcoin Price ‘Will Struggle’ In Big Financial Crisis, Says Investor (#GotBitcoin?)
Fidelity Charitable Received Over $100M In Crypto Donations Since 2015 (#GotBitcoin?)
Would Blockchain Better Protect User Data Than FaceApp? Experts Answer (#GotBitcoin?)
Just The Existence Of Bitcoin Impacts Monetary Policy (#GotBitcoin?)
What Are The Biggest Alleged Crypto Heists And How Much Was Stolen? (#GotBitcoin?)
IRS To Cryptocurrency Owners: Come Clean, Or Else!
Coinbase Accidentally Saves Unencrypted Passwords Of 3,420 Customers (#GotBitcoin?)
Bitcoin Is A ‘Chaos Hedge, Or Schmuck Insurance‘ (#GotBitcoin?)
Bakkt Announces September 23 Launch Of Futures And Custody
Coinbase CEO: Institutions Depositing $200-400M Into Crypto Per Week (#GotBitcoin?)
Researchers Find Monero Mining Malware That Hides From Task Manager (#GotBitcoin?)
Crypto Dusting Attack Affects Nearly 300,000 Addresses (#GotBitcoin?)
A Case For Bitcoin As Recession Hedge In A Diversified Investment Portfolio (#GotBitcoin?)
SEC Guidance Gives Ammo To Lawsuit Claiming XRP Is Unregistered Security (#GotBitcoin?)
15 Countries To Develop Crypto Transaction Tracking System: Report (#GotBitcoin?)
US Department Of Commerce Offering 6-Figure Salary To Crypto Expert (#GotBitcoin?)
Mastercard Is Building A Team To Develop Crypto, Wallet Projects (#GotBitcoin?)
Canadian Bitcoin Educator Scams The Scammer And Donates Proceeds (#GotBitcoin?)
Amazon Wants To Build A Blockchain For Ads, New Job Listing Shows (#GotBitcoin?)
Shield Bitcoin Wallets From Theft Via Time Delay (#GotBitcoin?)
Blockstream Launches Bitcoin Mining Farm With Fidelity As Early Customer (#GotBitcoin?)
Commerzbank Tests Blockchain Machine To Machine Payments With Daimler (#GotBitcoin?)
Man Takes Bitcoin Miner Seller To Tribunal Over Electricity Bill And Wins (#GotBitcoin?)
Bitcoin’s Computing Power Sets Record As Over 100K New Miners Go Online (#GotBitcoin?)
Walmart Coin And Libra Perform Major Public Relations For Bitcoin (#GotBitcoin?)
Judge Says Buying Bitcoin Via Credit Card Not Necessarily A Cash Advance (#GotBitcoin?)
Poll: If You’re A Stockowner Or Crypto-Currency Holder. What Will You Do When The Recession Comes?
1 In 5 Crypto Holders Are Women, New Report Reveals (#GotBitcoin?)
Beating Bakkt, Ledgerx Is First To Launch ‘Physical’ Bitcoin Futures In Us (#GotBitcoin?)
Facebook Warns Investors That Libra Stablecoin May Never Launch (#GotBitcoin?)
Government Money Printing Is ‘Rocket Fuel’ For Bitcoin (#GotBitcoin?)
Bitcoin-Friendly Square Cash App Stock Price Up 56% In 2019 (#GotBitcoin?)
Safeway Shoppers Can Now Get Bitcoin Back As Change At 894 US Stores (#GotBitcoin?)
TD Ameritrade CEO: There’s ‘Heightened Interest Again’ With Bitcoin (#GotBitcoin?)
Venezuela Sets New Bitcoin Volume Record Thanks To 10,000,000% Inflation (#GotBitcoin?)
Newegg Adds Bitcoin Payment Option To 73 More Countries (#GotBitcoin?)
China’s Schizophrenic Relationship With Bitcoin (#GotBitcoin?)
More Companies Build Products Around Crypto Hardware Wallets (#GotBitcoin?)
Bakkt Is Scheduled To Start Testing Its Bitcoin Futures Contracts Today (#GotBitcoin?)
Bitcoin Network Now 8 Times More Powerful Than It Was At $20K Price (#GotBitcoin?)
Crypto Exchange BitMEX Under Investigation By CFTC: Bloomberg (#GotBitcoin?)
“Bitcoin An ‘Unstoppable Force,” Says US Congressman At Crypto Hearing (#GotBitcoin?)
Bitcoin Network Is Moving $3 Billion Daily, Up 210% Since April (#GotBitcoin?)
Cryptocurrency Startups Get Partial Green Light From Washington
Fundstrat’s Tom Lee: Bitcoin Pullback Is Healthy, Fewer Searches Аre Good (#GotBitcoin?)
Bitcoin Lightning Nodes Are Snatching Funds From Bad Actors (#GotBitcoin?)
The Provident Bank Now Offers Deposit Services For Crypto-Related Entities (#GotBitcoin?)
Bitcoin Could Help Stop News Censorship From Space (#GotBitcoin?)
US Sanctions On Iran Crypto Mining — Inevitable Or Impossible? (#GotBitcoin?)
US Lawmaker Reintroduces ‘Safe Harbor’ Crypto Tax Bill In Congress (#GotBitcoin?)
EU Central Bank Won’t Add Bitcoin To Reserves — Says It’s Not A Currency (#GotBitcoin?)
The Miami Dolphins Now Accept Bitcoin And Litecoin Crypt-Currency Payments (#GotBitcoin?)
Trump Bashes Bitcoin And Alt-Right Is Mad As Hell (#GotBitcoin?)
Goldman Sachs Ramps Up Development Of New Secret Crypto Project (#GotBitcoin?)
Blockchain And AI Bond, Explained (#GotBitcoin?)
Grayscale Bitcoin Trust Outperformed Indexes In First Half Of 2019 (#GotBitcoin?)
XRP Is The Worst Performing Major Crypto Of 2019 (GotBitcoin?)
Bitcoin Back Near $12K As BTC Shorters Lose $44 Million In One Morning (#GotBitcoin?)
As Deutsche Bank Axes 18K Jobs, Bitcoin Offers A ‘Plan ฿”: VanEck Exec (#GotBitcoin?)
Argentina Drives Global LocalBitcoins Volume To Highest Since November (#GotBitcoin?)
‘I Would Buy’ Bitcoin If Growth Continues — Investment Legend Mobius (#GotBitcoin?)
Lawmakers Push For New Bitcoin Rules (#GotBitcoin?)
Facebook’s Libra Is Bad For African Americans (#GotBitcoin?)
Crypto Firm Charity Announces Alliance To Support Feminine Health (#GotBitcoin?)
Canadian Startup Wants To Upgrade Millions Of ATMs To Sell Bitcoin (#GotBitcoin?)
Trump Says US ‘Should Match’ China’s Money Printing Game (#GotBitcoin?)
Casa Launches Lightning Node Mobile App For Bitcoin Newbies (#GotBitcoin?)
Bitcoin Rally Fuels Market In Crypto Derivatives (#GotBitcoin?)
World’s First Zero-Fiat ‘Bitcoin Bond’ Now Available On Bloomberg Terminal (#GotBitcoin?)
Buying Bitcoin Has Been Profitable 98.2% Of The Days Since Creation (#GotBitcoin?)
Another Crypto Exchange Receives License For Crypto Futures
From ‘Ponzi’ To ‘We’re Working On It’ — BIS Chief Reverses Stance On Crypto (#GotBitcoin?)
These Are The Cities Googling ‘Bitcoin’ As Interest Hits 17-Month High (#GotBitcoin?)
Venezuelan Explains How Bitcoin Saves His Family (#GotBitcoin?)
Quantum Computing Vs. Blockchain: Impact On Cryptography
This Fund Is Riding Bitcoin To Top (#GotBitcoin?)
Bitcoin’s Surge Leaves Smaller Digital Currencies In The Dust (#GotBitcoin?)
Bitcoin Exchange Hits $1 Trillion In Trading Volume (#GotBitcoin?)
Bitcoin Breaks $200 Billion Market Cap For The First Time In 17 Months (#GotBitcoin?)
You Can Now Make State Tax Payments In Bitcoin (#GotBitcoin?)
Religious Organizations Make Ideal Places To Mine Bitcoin (#GotBitcoin?)
Goldman Sacs And JP Morgan Chase Finally Concede To Crypto-Currencies (#GotBitcoin?)
Bitcoin Heading For Fifth Month Of Gains Despite Price Correction (#GotBitcoin?)
Breez Reveals Lightning-Powered Bitcoin Payments App For IPhone (#GotBitcoin?)
Big Four Auditing Firm PwC Releases Cryptocurrency Auditing Software (#GotBitcoin?)
Amazon-Owned Twitch Quietly Brings Back Bitcoin Payments (#GotBitcoin?)
JPMorgan Will Pilot ‘JPM Coin’ Stablecoin By End Of 2019: Report (#GotBitcoin?)
Is There A Big Short In Bitcoin? (#GotBitcoin?)
Coinbase Hit With Outage As Bitcoin Price Drops $1.8K In 15 Minutes
Samourai Wallet Releases Privacy-Enhancing CoinJoin Feature (#GotBitcoin?)
There Are Now More Than 5,000 Bitcoin ATMs Around The World (#GotBitcoin?)
You Can Now Get Bitcoin Rewards When Booking At Hotels.Com (#GotBitcoin?)
North America’s Largest Solar Bitcoin Mining Farm Coming To California (#GotBitcoin?)
Bitcoin On Track For Best Second Quarter Price Gain On Record (#GotBitcoin?)
Bitcoin Hash Rate Climbs To New Record High Boosting Network Security (#GotBitcoin?)
Bitcoin Exceeds 1Million Active Addresses While Coinbase Custodies $1.3B In Assets
Why Bitcoin’s Price Suddenly Surged Back $5K (#GotBitcoin?)
Zebpay Becomes First Exchange To Add Lightning Payments For All Users (#GotBitcoin?)
Coinbase’s New Customer Incentive: Interest Payments, With A Crypto Twist (#GotBitcoin?)
The Best Bitcoin Debit (Cashback) Cards Of 2019 (#GotBitcoin?)
Real Estate Brokerages Now Accepting Bitcoin (#GotBitcoin?)
Ernst & Young Introduces Tax Tool For Reporting Cryptocurrencies (#GotBitcoin?)
Recession Is Looming, or Not. Here’s How To Know (#GotBitcoin?)
How Will Bitcoin Behave During A Recession? (#GotBitcoin?)
Many U.S. Financial Officers Think a Recession Will Hit Next Year (#GotBitcoin?)
Definite Signs of An Imminent Recession (#GotBitcoin?)
What A Recession Could Mean for Women’s Unemployment (#GotBitcoin?)
Investors Run Out of Options As Bitcoin, Stocks, Bonds, Oil Cave To Recession Fears (#GotBitcoin?)
Goldman Is Looking To Reduce “Marcus” Lending Goal On Credit (Recession) Caution (#GotBitcoin?)
Your Questions And Comments Are Greatly Appreciated.
Monty H. & Carolyn A.
Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,Treatments, Vaccines And A,
Go back


Leave a Reply
You must be logged in to post a comment.