Fighting Cancer By Releasing The Brakes On The Immune System
Cancer immunotherapy comes in several forms. The drugs sparking the most interest are called checkpoint inhibitors. Fighting Cancer By Releasing The Brakes On The Immune System
They work by releasing the natural brakes on the immune system, enabling its foot soldiers, called T cells, to attack tumors.
“It’s the most exciting thing I’ve ever seen,” says David Lane, scientific director of New York’s Ludwig Institute for Cancer Research. “It’s the long-term survival of people who have advanced disease. This is very unusual.”

It is hard to know how many patients whose cancers have metastasized, or spread, have enjoyed sustained survival following immunotherapy treatment. An analysis of 4,846 advanced melanoma patients treated with one checkpoint inhibitor— Bristol-Myers Squibb Co. ’s Yervoy—found that 21% were still alive three years later. That amounts to more than 1,000 people, most of whom experts say almost certainly would have died otherwise. Especially striking is how good the long-term prospects were for people who survived at least three years.

“The people that make it after three years don’t die of melanoma,” says James Allison, head of immunology at MD Anderson Cancer Center in Houston, whose seminal discovery about the immune system and cancer in the mid-1990s laid the groundwork for many of the current advances.
Newer drugs that work similarly to Yervoy, but on different immune-system brakes, are getting even better early results and are extending the benefits beyond melanoma to other cancers.
Immunotherapy is still in its early stages, and more rigorous studies are needed. Oncology is filled with tales of advances that held promise only to be thwarted by cancer’s uncanny ability to develop resistance to medicine’s attacks. There isn’t any assurance that the new immunotherapy will be different.
Researchers and drug makers are striving to overcome huge obstacles to a lasting cure. For one, most patients don’t respond the way the super-survivors have, and researchers are just beginning to understand why. Another mystery is why some patients relapse while remaining on therapy while others go into prolonged remission after undergoing just one course of treatment.
Most experts believe it will take combinations of immunotherapy drugs—or combinations of immunotherapy with other cancer treatments—to optimize their impact. But finding safe and effective combinations is a daunting undertaking.
While side effects of the new drugs are relatively mild for some patients, others have developed potentially devastating complications caused by an out-of-control immune system. Some patients have died as a result. Researchers are devising ways to minimize such problems.
“It’s extremely exciting that so many patients are responding” to checkpoint inhibitors, says Bert Vogelstein, director of the Ludwig Center at Johns Hopkins Kimmel Cancer Center in Baltimore. “But the reality is that most are not.”
The drugs, which are costly to develop, are certain to fuel the debate about the cost of innovative drugs. Yervoy costs more than $120,000 for a four-course treatment, while Merck & Co’s Keytruda, approved in September for advanced melanoma, costs $12,500 a month, or $150,000 for a year.
More than 25 companies ranging from the pharmaceutical industry’s biggest names to a group of startups are pursuing some form of immunotherapy.
Bristol-Myers’s drug Yervoy, which is based on Dr. Allison’s discoveries, blocks an immune system brake called CTLA-4. Merck’s Keytruda inhibits a brake called PD-1. Bristol, Roche Holding AG and AstraZeneca PLC are among several companies also testing agents against checkpoint targets.
Another approach involves genetically modifying certain T cells outside the body, creating what are called CAR T cells, and infusing them back to attack targets on the surface of cancer cells.
Novartis AG , closely held Juno Therapeutics Inc., Kite Pharma Inc., and a collaboration between Bluebird bio and Celgene are pursuing this strategy. Amgen Inc. is developing yet another T-cell approach while several other companies are reviving efforts to develop cancer vaccines.
“I divide pharmaceutical companies into two categories,” says Drew Pardoll, co-director of immunology at Johns Hopkins. “They’re in immunotherapy up to their eyeballs, or they want to be.”
The efforts are a departure from most current cancer treatments, including chemotherapy, radiation and the new crop of medicines that target genetic mutations underlying a tumor’s growth. These strategies take direct aim at the tumor—generally to only limited effect in patients with advanced cancer. Even the recent excitement about genetically targeted drugs has been tempered by the ability of tumors to mutate and grow resistant to tailored attacks.
With immunotherapy, “We’re treating the immune system, not the cancer,” says Dr. Allison.

Richard Logan, a 59-year-old veterinarian in Ozark, Ala., embraced the alternative approach in 2009, soon after melanoma that had started as a bleeding mole on his back progressed to his lung and liver. He already had endured about a year of treatment with interferon, an immune-system booster with harsh side effects that left him exhausted.
In addition, his father had recently died of melanoma, succumbing about six months after the cancer had spread from its original site. “I pretty much knew the old treatments weren’t going to be much help,” he says.
An Internet search led him to the melanoma center at Boston’s Dana Farber Cancer Institute. In July 2009 he enrolled in a trial there for a drug that later became Yervoy. Within two months, his tumors started shrinking and by that December, he says, “I felt pretty confident I was going to have extended relief from this disease.”
Now nearly five years later, Dr. Logan says his cancer has stabilized. He remains on Yervoy, flying regularly to Boston for infusions. He rates the side effects on Yervoy as a “two or three” compared with a 10 on interferon. He continues to run his veterinary practice, saw his son graduate from college and recently remarried.
He doesn’t use the word “cure” to describe his status, saying only that he is “confident” in how he is doing on the drug. “If this starts to pale, I’m keeping aware of whatever else is available out there,” he says. “I’m pretty even-keel and take it as it comes.”
Getting the immune system to “see” and attack tumors has stymied researchers for decades. Unlike, say, a flu virus—an invader that quickly catches the immune system’s attention—a tumor cell may reflect only “modest changes in cells that the body has been taught to leave alone and tolerate,” says Walter J. Urba, director of cancer research at Providence Cancer Center in Portland, Ore. Moreover, he says, “cancer cells are pretty smart and they change in ways that can avoid the immune system.”

For years, scientists thought the immune system didn’t recognize tumors at all. Then research on biopsy specimens revealed that T cells often succeed in infiltrating the environment around tumors, but either fail to mount an adequate response or hold the cancer at bay for years before finally being overmatched.
When the T cell does recognize the tumor, it amounts to an ignition switch for the immune response. But just “turning the key” isn’t enough. T cells generally need a second so-called co-stimulatory signal to become activated against the cancer. That is the gas pedal.
For two to three decades, immunotherapy efforts have focused on the gas pedal. Researchers and companies have tested vaccines that educate T cells to find cancer cells and developed treatments to boost activated T cells and direct them at vulnerable targets on tumors. Despite some success against relatively rare cancers, results have proved lackluster.

Sharon Belvin was diagnosed with advanced melanoma 10 years ago, two weeks before her wedding. After chemotherapy, radiation and drug therapy failed to stop the cancer, she enrolled in a clinical trial for a new immunotherapy drug, now known as Yervoy. One year later, all evidence of her cancer had disappeared from scans. She now has two children and is a personal trainer.
“No matter what you did, only 10% to 15% of patients seemed to get a benefit,” says F. Stephen Hodi, director of the melanoma center at Dana Farber.
The insight that changed the equation came from Dr. Allison. In the early 1990s, researchers racing to unravel how T cells function homed in on a molecule called CTLA-4. They had suspected it was a “gas pedal” that activated the immune system. Dr. Allison, then at University of California Berkeley, was among those who determined that it actually inhibited the immune response—it was a brake.
That led him to a question: Would blocking CTLA-4 with a drug—in effect, suppressing the suppressor—unleash the immune system to attack cancer?
He and his colleagues fashioned an antibody to CTLA-4. In a study published in Science in 1996, they showed that the strategy, which prevented the immune system from turning off, led to the eradication of tumors in mice.
Translating that discovery into an FDA-approved medicine took 15 years. After shopping the antibody to skeptical drug companies for two years, Dr. Allison joined forces with a small company called Medarex Inc., then in Princeton, N.J. The initial human studies were encouraging enough to draw Bristol-Myers into a collaboration with Medarex to mount a large-scale study.
Early results were less than impressive. Tumors shrank sufficiently in only about 10% of patients—no better than was seen in other forms of immunotherapy, raising doubts the drug could be approved.
But researchers also noticed something unusual: Some patients were living much longer than expected, including some who had gone off therapy. Others reported feeling better even though their tumors hadn’t quickly responded by conventional measures.
One of them was Mr. Telford, the high-school teacher with advanced melanoma. In June 2006, with the encouragement of Jedd Wolchok, chief of melanoma and immunotherapy at Sloan Kettering, he enrolled in a study of the Bristol-Medarex anti-CTLA-4 drug. The protocol called for four treatments, one every three weeks. The goal: tumor shrinkage at 12 weeks.
It didn’t work. After Mr. Telford’s last infusion, his scans showed tumors in his liver had gotten much larger. Dr. Wolchok prepared to deliver the message oncologists dread: The drug wasn’t working and there weren’t any other options.
But in the exam room, Mr. Telford told the doctor: “This is the best I’ve felt in months.”
When Dr. Wolchok insisted the scans showed no improvement, Mr. Telford recalls saying, “I don’t care what your scans say, I feel better.” His energy levels were up and his night sweats had stopped.
A mystified Dr. Wolchok told him to come back for another checkup in two months. This time, the tumors were getting smaller. By May 2007, his scans showed no evidence of cancer.
Mr. Telford remained on the drug until last December. He is essentially free of disease. Now 60 years old, he is still teaching and coaching baseball.
So exceptional was Mr. Telford’s case that he became known among immunotherapy researchers as “the liver guy,” Dr. Wolchok says. His experience “was a pivotal moment” that helped prompt Medarex and Bristol-Myers to change the primary measurement of the trial, Dr. Wolchok says. Instead of looking at how long the therapy kept cancer from progressing, a measure that can lead to approval of drugs for metastatic cancer, they opted to test for overall survival.
It took some 700 patients and extended the trial to 2010. In the end, fewer than 10% met standard criteria for tumor shrinkage. But 23% survived at least three years, making the drug the first to ever show a survival benefit in patients with advanced melanoma. The drug, now owned by Bristol-Myers and known as Yervoy, was approved in 2011.
The challenge was to widen the benefit to more patients and more cancers.
For reasons of biology, melanoma is more vulnerable than most other tumor types to attacks by the immune system. For immunotherapy to become a mainstay of cancer treatment, it needs to prove effective against more tumors.
That is beginning to happen. Data presented at the American Society of Clinical Oncology meeting in June and at the European Society of Medical Oncology in September offered encouraging results for bladder, head and neck, kidney and other cancers. Checkpoint inhibitors are being tested in breast and pancreatic cancers, and Hodgkin lymphoma, multiple myeloma and other cancers.
The disease attracting intense interest is lung cancer, which causes more than 200,000 deaths a year in the U.S.
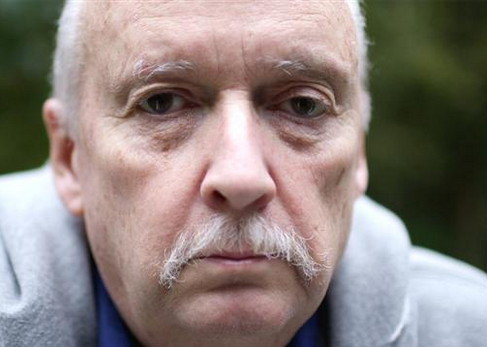
David Gobin, a retired Baltimore policeman, was diagnosed with lung cancer at 58. He lost much of his right lung to surgery and much of his spirit to aggressive chemotherapy, which failed.
In late 2010, he joined a trial at Johns Hopkins for an experimental Bristol-Myers drug that blocks the immune system brake called PD-1. The protocol called for a one-hour infusion every two weeks for two years.
Four months later, a scan showed his tumor had significantly improved. Remnants of the tumor still showed up on scans when his treatment ended after two years, but it hasn’t grown since.
“Every time we scanned him, things were decreasing,” says Julie Brahmer, his oncologist at Hopkins. “With the rate his disease was progressing back when I first met him, he shouldn’t be around.”
Mr. Gobin gets short of breath because of the lung surgery, and he needs to take frequent rests. He isn’t complaining.
Before being treated with the drug—now called nivolumab—he was twice told he had less than a year to live. He hasn’t taken the drug for 23 months.
“I still have a little cancer. It’s still sitting there,” he says. “It’s not doing anything.”
Most researchers believe that the key to expanding immunotherapy lies in combination treatments.
A 53-patient Bristol-Myers study combining Yervoy and nivolumab resulted in a two-year melanoma survival rate of 79%, but that came at a cost of serious side effects.
Researchers also are experimenting with pairing such drugs with vaccines or with treatments such as chemotherapy, radiation and genetically targeted drugs. The hope is that attacking the tumor with these approaches will make it more visible to T cells, a necessary step if releasing the brakes is to have a significant effect on the cancer.
“Almost every combination is appealing in some way,” says Roy Herbst, chief of medical oncology at Yale Cancer Center, but “how to sort through all that is incredibly difficult.”

Richard Murphy, a father of three from Marshfield, Mass., didn’t know what a biopsy was when he was diagnosed in 2008, at 43, with a rare form of melanoma. He underwent two surgeries and several bouts of radiation and chemotherapy, but his tumors spread to his lung and surrounded his spine. He enrolled in a study of Yervoy in February 2011.
After his fourth treatment at Dana Farber, scans showed his tumors had shrunk. But by August, he says, “everything kind of had grown.” Dr. Hodi soon concluded the drug wasn’t working and took him off the study.
Dr. Hodi had another idea. His colleagues at Dana Farber had played key roles in identifying PD-1 as an immune checkpoint, and a slot opened up in a study of an anti-PD-1 agent being developed by Merck that became known as Keytruda. In March 2012, Mr. Murphy had his first dose.
The day of his scheduled sixth treatment in June, a blood test showed “off-the-chart” levels of an enzyme that suggested possible muscle damage. He was hospitalized out of concern for possible kidney failure. Whether the PD-1 drug was responsible isn’t certain, but Dr. Hodi took him off the study.
The next day, an ultrasound to check his kidneys revealed something else: Mr. Murphy’s tumors were shrinking. By the end of August 2012, there wasn’t any evidence of disease on his scans, just shadows where tumors had been. His checkup this October showed the same.
Mr. Murphy, a real-estate agent, stages and participates in triathlons to raise money for cancer research.
“I was on two clinical trials and I was kicked off two clinical trials,” he says. “I don’t think you’d expect the outcome that we have. You wouldn’t expect that would be the pot of gold.”
Updated: 1-2-2015
Israeli Vaccine Blocks 90% of Cancer Types
Vaxil BioTherapeutics’s ImMucin trains immune system to fight cancer cells and prevent the disease’s return for early stages and remission.
An Israeli biotech company is developing a vaccine for cancer that it says can help prevent the return of the lethal disease for 90% of the different types of cancer.
Vaxil BioTherapeutics based in Nes Ziona has been developing ImMucin for more than five years, and already has seen strong success in testing indicating it can be a vital tool in combating cancer. The disease kills eight million people worldwide per year, and sees 14 million new cases diagnosed annually according to the World Health Organization.
“Vaxil is developing a drug to keep the cancer from coming back,” Vaxil’s CFO Julian Levy told NoCamels. “We are trying to harness the natural power of the immune system to fight against cancer by seeking out cancer cells and destroying them.”
ImMucin is not intended to replace chemotherapy or other traditional cancer treatments, but rather is meant to halt the development of the disease at less intense periods of the process, during early stages of detection and remission.
Remarking on the concept of a cancer vaccine, Levy said “many preventative cancer vaccines today are not actual vaccines against cancer. Young women can take a vaccine for the HPV virus, which doesn’t combat cancer; it’s a vaccine against a virus that has been proven to lead to a more serious cervical cancer.”
So How Does ImMucin Work?
The vaccine stimulates a certain part of the immune system, developing it to attack specific cells with markers indicating cancer. When used in early stages of cancer, the vaccine is expected to train the immune system to destroy the right cells as cancer develops and fight the disease.
Until last January the company focused its experiments on Multiple Myeloma patients, but then shifted to breast cancer patients. It may be some time before the vaccine sees its way onto the market though, with Levy expecting a release by 2020 at the latest.
Updated: 2-18-2016
Black Female Physicist Pioneers Technology That Kills Cancer Cells With Lasers
Dr. Hadiyah-Nicole Green is one of fewer than 100 black female physicists in the country, and the recent winner of $1.1 million grant to further develop a technology she’s pioneered that uses laser-activated nanoparticles to treat cancer.
Green, who lost her parents young, was raised by her aunt and uncle. While still at school, her aunt died from cancer, and three months later her uncle was diagnosed with cancer, too.
Green went on to earn her degree in physics at Alabama A&M University, being crowned Homecoming Queen while she was at it, before going on full scholarship to University of Alabama in Birmingham to earn her Masters and Ph.D.
There Green would become the first to work out how to deliver nanoparticles into cancer cells exclusively, so that a laser could be used to remove them, and then successfully carry out her treatment on living animals.
As she takes on her growing responsibilities, Green still makes time to speak at schools, Boys & Girls Clubs and other youth events. “Young black girls don’t see those role models (scientists) as often as they see Beyonce or Nicki Minaj,” says Green. “It’s important to know that our brains are capable of more.”
A Physicist’s Cancer Treatment
A few months ago, Green was awarded a $1.1 million grant to work on a technology that targets, images and treats cancer.
I’m no different from anybody else. When opportunity found me, I was prepared.
“I was completely overwhelmed with joy, with thanksgiving, humbled at the opportunity that a group of my peers thought that my work was worthy for such a grant,” she said. “This is a huge door opening. It outlines a path to take this treatment to clinical trial.”
Green had spent seven years during her master’s and doctoral programs at UAB, developing a way to target cancer cells – not the healthy cells around them.
“I’m really hoping this can change the way we treat cancer in America,” said Green. “There are so many people who only get a three-month or six-month survival benefit from the drugs they take. Then three or six months later, they’re sent home with no hope, nothing else we can do. Those are the patients I want to try to save, the ones where regular medicine isn’t effective for them.”
The way the technology works is that an FDA-approved drug containing nanoparticles is injected into a cancer patient and causes the patient’s tumor to fluoresce (glow) under imaging equipment. The goal is for a laser to activate the nanoparticles by heating them.
“They are not toxic, so without the laser they won’t kill anything, and the laser by itself is harmless, so without the particles it won’t hurt anything,” said Green. “Because of their need to work together and their inability to work apart, I can insure that the treatment is only happening to the cancer cells we target and identify.”
While Green is not the first to think of using lasers and nanoparticles to treat cancer, she’s been able to work the bugs out of parts of the technology that have been problematic, like nanoparticle delivery and seeing success in living animals – mice, in Green’s case.
“As a physicist I’ve created a physical treatment that is not specific to the biology of the cancer,” she said. “It’s a platform technology. It’s not cancer type-specific, though it can treat the cancer specifically. That’s a concept my friends who are biologists struggle with.”
Capable Of More
As she moves forward with her research and with teaching at Tuskegee, Green makes time to speak at schools, Boys & Girls Clubs and other youth events.
“People told me to make good grades and stay in school,” she said, “and I always take good advice to heart.”
Green said she feels a responsibility to be a positive example and change stereotypes of black women portrayed in media.
“There are black female scientists who don’t get media exposure,” she said. “Because of that, young black girls don’t see those role models as often as they see Beyonce or Nicki Minaj. It’s important to know that our brains are capable of more than fashion and entertainment and music, even though arts are important.”
Green has mentored several young women, many of whom have gone on to receive degrees and jobs in science-related fields.
“It takes a village to raise a child,” she said. “I repeat that because a village of people helped raise me and instill values in me, and encouraged me to get to this point. I did not get here by myself. Because of that clarity, I know my responsibility to encourage and mentor the next generation.”
Updated: 7-27-2020
AstraZeneca Strikes $6 Billion Cancer Drug Deal
Therapy is designed to leave healthy cells alone, potentially limiting side effects.
AstraZeneca PLC has agreed to pay Japan’s Daiichi Sankyo Co. up to $6 billion to jointly develop and commercialize a cancer drug it says could help redefine the way the disease is treated, in the British company’s latest push into oncology.
The therapy, an antibody drug conjugate named DS-1062, targets a range of cancers—including lung and breast—that produce a protein known as TROP2. It is designed to deliver chemotherapy just to those cells, leaving healthy ones alone, potentially limiting side effects.
Under the deal announced Monday, AstraZeneca will pay Daiichi Sankyo $1 billion upfront, an additional $1 billion dependent on regulatory approvals and up to a further $4 billion for sales-related milestones.
The companies will equally share costs and profits from the drug, except in Japan, where Daiichi Sankyo retains exclusive rights and will be responsible for costs and pay AstraZeneca royalties.
AstraZeneca cautioned that while it sees significant potential in DS-1062, the drug has yet to be approved anywhere, and its safety and efficacy are yet to be established.
While AstraZeneca’s profile has been raised this year because of its work developing a coronavirus vaccine with the University of Oxford, one of its key focuses in recent years has been pushing further into cancer treatments.
The company is betting that a sharper focus on oncology can revive its fortunes after a series of patent expirations on bestselling drugs like cholesterol pill Crestor knocked billions of dollars off its revenue.
AstraZeneca has launched six new cancer medicines since 2014 and last year hired a prominent cancer doctor, José Baselga, to lead oncology research and development. It signed a deal worth up to $6.9 billion last year for shared rights to another cancer drug with Daiichi Sankyo.
AstraZeneca Chief Executive Pascal Soriot said Monday the company now had six potential blockbuster treatments in oncology and that more could come from its early and late-stage pipeline.
Developing cancer drugs is an increasingly competitive area as the world’s biggest pharmaceutical companies seek to commercialize scientific advances. Rivals including Pfizer Inc. and GlaxoSmithKline PLC are also focusing more on oncology.
Updated: 9-20-2021
AstraZeneca Breast Cancer Drug Found To Reduce Risk Of Dying
Large trial shows Enhertu lowers risk of death or tumor progression.
AstraZeneca PLC said its breast cancer drug Enhertu significantly reduced the risk of dying or disease progression in women with advanced disease in a large clinical trial, the latest sign that its push into oncology is starting to pay off.
For women with metastatic breast cancer, an advanced form of the disease where tumors have spread to other parts of the body, Enhertu reduced the risk of death or tumor progression by 72% compared with Kadcyla, the current standard treatment, the trial found.
The phase-three trial, called DESTINY-Breast03, compared Enhertu with Kadcyla in around 500 women whose tumors produce high levels of a protein called HER2 and whose cancers haven’t responded to earlier treatment. Kadcyla, made by Roche Holding AG, is the current standard of care in these patients.
“We’ve never seen a magnitude of benefit like this in metastatic breast cancer before,” said Dave Fredrickson, AstraZeneca’s head of oncology.
AstraZeneca, which along with Oxford University developed one of the world’s most widely used Covid-19 vaccines, has invested heavily in cancer medicine in recent years. Like other drug makers, it has been drawn to oncology by a string of scientific breakthroughs and the promise of high returns.
The company bet big on Enhertu, having acquired the shared rights to the drug through a $6.9 billion deal with Japan’s Daiichi-Sankyo Co., its original developer, in 2019.
In addition to treating patients with advanced cancer, AstraZeneca hopes the drug could also be used to treat, and potentially cure, early-stage disease.
The results, shared at a medical conference Saturday, suggest that Enhertu could have further success in earlier stages of treatment and other forms of cancer, said Jefferies analyst Peter Welford. He and others predict that Enhertu could potentially generate billions of dollars in yearly sales for AstraZeneca.
Enhertu is currently used as a so-called third-line treatment in women with advanced HER2-positive breast cancer. That means it is used after two previous forms of treatment have failed to stop disease progression. The latest results will pave the way for Enhertu to be used earlier on in treatment.
Enhertu works by tracking down cancer cells in the body and delivering a dose of chemotherapy at the site of the tumors. In that way, it leaves healthy tissue alone, in contrast with traditional chemotherapy that cannot differentiate between tumor and normal cells.
In the trial, 75.8% of women treated with Enhertu had no disease progression one year into treatment. Of the women treated with Kadcyla, 34.1% had no disease progression one year on.
Javier Cortés, head of the International Breast Cancer Center in Barcelona and one of the trial’s lead investigators, called the results remarkable.
He said that patients with previously treated HER2-positive breast cancer typically experience disease progression in less than a year with the currently available treatments and that the results would support the potential of Enhertu to become the new standard of care for these patients.
Updated: 11-24-2021
All Those 23andMe Spit Tests Were Part Of A Bigger Plan
CEO Anne Wojcicki wants to make drugs using insights from millions of customer DNA samples, and doesn’t think that should bother anyone.
A few months ago, on the morning 23andMe Holding Co. was about to go public, Chief Executive Officer Anne Wojcicki received a framed sheet of paper she hadn’t seen in 15 years.
As she was preparing to ring in the Nasdaq bell remotely from the courtyard of her company’s Silicon Valley headquarters, Patrick Chung, one of its earliest investors, presented her with the pitch document she’d shown him when she was first asking for money, reproduced on two pieces of paper so she could see both sides. The one-sheet outlined a radical transformation in the field of DNA testing.
Wojcicki’s plan back then was to turn genetics from the rarefied work of high-end labs into mainstream health and quasi entertainment products.
First she’d sell tastemakers on her mail-in spit kits as a way to learn sort-of-interesting things about their DNA makeup, such as its likely ancestral origins and the chance it would lead to certain health conditions.
Eventually she’d be able to lower prices enough to make the kits broadly accessible, allowing 23andMe to build a database big enough to identify new links between diseases and particular genes.
Later, this research would fuel the creation of drugs the company could tailor to different genetic profiles. 23andMe would become a new kind of health-care business, sitting somewhere between a Big Pharma lab, a Big Tech company, and a trusted neighborhood doctor.
Some of this still sounds as far off now as it did during the Bush years. Improbably, though, 23andMe has rounded second base and is heading for third. Wojcicki did sell millions of people on DNA test kits—11 million and counting—and bring such tests to the mainstream, with some help from Oprah’s holiday gift guide.
An estimated 1 in 5 Americans have turned over their genetic material to 23andMe or one of its competitors. Now that she’s got the data, Wojcicki is working on the drugs.
Her company is collaborating on clinical trials for one compound (and nearing trials for another) that could be used for what’s known as immuno-oncology, treatments that attempt to harness the body’s complex immune system to beat cancer.
23andMe says it’s also exploring drugs with potential use in treatments for neurological, cardiovascular, and other conditions, though it declined to specify them.
Last month the company bought Lemonaid Health, a telehealth and drug delivery startup that offers treatment and prescriptions for a select group of conditions, including depression, anxiety, and STDs.
Chung, now a co-founder and general partner at the venture capital firm Xfund, says his framed gift to Wojcicki was meant to emphasize how exceptionally closely she stuck to her original vision for 23andMe over the past decade and a half.
Most young tech companies in search of their initial venture funding have little hope of identifying their eventual path to great wealth and instead focus their energies on some sort of “dancing startup laser light show,” he says.
“The rule, not the exception, is that whatever the founders are pitching at the very earliest stages is simply never the thing that is successful.” 23andMe went public in June at a market value of $3.5 billion, is now closer to $5 billion, and is expected to report about $56 million in revenue in its latest quarter, though it’s unlikely to turn a profit.
Of course, Wojcicki, a former Wall Street research analyst with a focus on biotech, did have some experience with outstanding startups. Soon after she closed that round of venture funding, she married longtime boyfriend Sergey Brin, who co-founded Google in her sister Susan’s garage. (Wojcicki and Brin divorced in 2015.)
It’s easy to see the Google influence in 23andMe’s strategy—collect all the data, derive whatever insights you can, and find an adjacent line of business with the potential to yield much bigger profits.
Even today, Wojcicki’s conversations tend to be shot through with an older strain of Web 2.0 techno-optimism about ways to better connect people with, in this case, how their medicines get made, and to cut through the randomness and waste that suffuses the science of drugmaking.
“One of our core values is, like, we’re all in this together,” she says. “One thing I always think is a tragedy is that you develop a drug and then people hate you. I’m really interested in, can we actually be the first drug development group that is loved by people?”
The next phase of her master plan might sorely test that question. While it’s difficult to imagine anyone saying they love their pharmaceutical company, it wouldn’t be crazy for the 8.8 million 23andMe customers who once absently checked a box saying yeah, sure, use my data for whatever, to feel like they’ve been bait-and-switched now that their genes are laying the groundwork for potential cancer cures.
Privacy advocates have been warning for years that the spit-tube deal is lopsided—that there aren’t enough legal protections on genetic data to justify trading DNA samples for answers about whether you’re predisposed to hate cilantro or what percent Swedish you are. DNA data, which contains information about you and your blood relatives, could be hacked, de-anonymized, or shared with the police.
23andMe’s pharma ambitions add a new dimension to these concerns. If Wojcicki keeps achieving her goals, customers might one day pay 23andMe to assess their disease risk and pay for a treatment it later develops based on their DNA.
Why should one company hold the key to the world’s genetic code—and charge the rest of us handsomely for access to it?
And before 23andMe reaches that threshold, it will have to deal with the reality that Big Pharma is hard. The company is trying to expand from a category of modest consumer products into a field full of goliaths a couple of orders of magnitude larger, with research and development operations that dwarf anything it’s fielding.
“Genetics is just one little piece of the puzzle,” says Harvard geneticist Robert Green. “So far there have only been a few instances where genetics has been the key to a brand-new drug. It’s not yet clear it’s going to have the value we’re hoping it does.”
For now, Wojcicki’s answer to both sets of concerns is, basically, Trust Us. “I always like to say, ‘We’ll win you over,’ ” she says.
From the moment the first draft of the human genome was published in 2001, scientists have evangelized the potential of genetic data to discover and develop drugs. That’s because Big Pharma’s process, for all the billions of dollars in annual R&D spending, remains a crapshoot.
The mysteries of human biology mean that, more often than not, promising drug candidates, even those that make it deep into clinical trials, wind up not working. The industry calls that period between the lab and the market the Valley of Death. An estimated 13% of all global drug development programs end in approvals.
Even then, drugs can inexplicably work well for some people and not others. To this day, researchers sometimes can’t explain the molecular mechanisms that underpin common medications.
Given enough data, genetics can identify patterns that provide significant clues. When you’re hunting for what might be a few letters of variation among 3 billion pairs of DNA nucleotides, scale is what matters.
Often, many genes contribute to a particular condition to varying degrees, so more examples give researchers more chances to puzzle out how they fit together.
Wojcicki and her co-founders weren’t the first to think of building a database for this purpose, but using consumer DNA kits as an input soon gave her company a massive scalar advantage over pretty much everyone.
(The only bigger known databases belong to Ancestry.com and the Chinese government.) 23andMe started selling its kits in 2007, pitching them mostly as a way to assess a smattering of health traits, such as a customer’s risk of colorectal cancer or likelihood of lactose intolerance, along with a rudimentary version of its ancestry analysis.
Criticism soon followed. One article, published in the journal BMJ in 2008, argued that “rather than improving health, widespread genetic testing is likely to result in widespread anxiety.”
On these sorts of grounds, the U.S. Food and Drug Administration forced the company to stop selling its tests without approval in 2013. Other critics questioned whether consumers could self-report their own health data accurately.
23andMe relies on customers to help its analysis along by flagging known health issues—whether a given person has a heart condition, for example, or an autoimmune disorder.
Eventually, with copious peer-reviewed research, 23andMe won over the scientific community and the FDA, which allowed it to resume selling the tests in a pared-down form in 2015.
Today the company sells an ancestry test for $99, one with health “insights” for $199, and a subscription service that includes reports on heart health and a person’s particular response to different medications.
For most Silicon Valley companies, hoarding the test-kit data and selling it to drugmakers would have been the endgame. Instead, 23andMe is trying to make the drugs, too.
The company’s 100 staff scientists, hired over the past six years, work out of its therapeutics offices in South San Francisco, half an hour north of its headquarters in Sunnyvale, Calif.
While the Sunnyvale campus is all glass and contemporary opulence, the therapeutics lab is a drab, squat complex with a 1980s flavor. (On the other hand, it’s tough to knock the sweeping bay views.) The scientists worked through the pandemic here, at lab benches spaced for social distancing, with thick cables running from the benchtops to the ceiling.
The facility is divided roughly into halves, each with its own offices and labs, by a big room for shipping and receiving and an even bigger set of storage chambers for gases, chemicals, and distilled water.
On one side, robots make the drug prototypes. There are robots for pipetting DNA into cells, robots for purifying liquids, and even robots that just swirl containers of liquid for a certain amount of time.
On the other side, scientists suss out the basics of how genes and diseases are connected, often performing tasks by hand at their benches.
Aside from the research scientists, the staff is mostly working remotely, and the rows of empty cubicles lend the more corporate parts of the office the feeling of a startup in the aftermath of the dot-com bust.
The labs, though, have remained busy. During the company’s last fiscal year, from April 2020 to March 2021, it roughly doubled its number of good potential drug candidates, as evaluated by its scientists, to 19.
Their focus: diseases that 23andMe has lots of data on, that many people suffer from, and that lack good treatment options. “We prioritize based on power, need, and speed,” says Kenneth Hillan, head of the therapeutics division.
Every six months or so—after, say, a big post-holidays influx of customers—23andMe’s computational biologists scan the company database looking for variations that, based on correlations in the company’s massive database of human DNA, appear more common among people with particular diseases.
Turning that hint of where there might be treasure into a map where X marks the spot takes many months of research in the dreary building full of scientists. But the database has the potential to narrow to months a process that otherwise might take many years.
While pharma companies typically specialize in a few kinds of treatments, 23andMe can instead direct its research wherever the data takes it, and it has a ready supply of possible future clinical-trial participants and customers. The end result is the potential to develop drugs more quickly and efficiently than the status quo.
This has made it an attractive partner to traditional drugmakers, notably GlaxoSmithKline Plc, which took a $300 million stake in 23andMe in 2018. Within a year, GSK and 23andMe already had six potential targets in the works. One candidate, a cancer drug that works to block CD96, a protein that helps modulate the body’s immune responses, entered clinical trials in four years. The industry average is closer to seven.
In the case of the drug 23andMe is developing in-house, scientists discovered a biological pathway, or series of molecular actions that lead to a change in a cell, strongly associated with the way immune cells attack a certain kind of tumor.
The drug is designed to block the tumor-induced suppression of the immune system’s T cells and then reactivate the immune system’s response to the tumor.
Sometimes the database can also highlight new uses for existing drugs. “The size has opened up the opportunity to be able to see genetic associations that you can’t see anywhere else because you need the math of the numbers to be able to see it,” says John Lepore, GSK’s head of research. At one point, a genome-wide association study showed that multiple genes within a particular pathway appeared to be linked to asthma.
There was already an antibody drug in development for asthma by another company that works by blocking the action of that pathway. 23andMe scientists were able to further interrogate the database to find that drugging that pathway might also help to treat a certain rare disease.
Wojcicki is now gearing up to shift more toward solo projects. That was a big reason why 23andMe went public in June, via a merger with VG Acquisition Corp., a special purpose acquisition company, or SPAC, founded by billionaire Richard Branson.
By March of next year it expects to have its first drug developed fully in-house in clinical trials, and it says its Phase I trial with GSK should return data by the end of 2022.
Skeptics, however, argue it’s unrealistic to expect the company to be great at drug development right from the start, especially as it decouples its efforts from Glaxo and other experienced developers.
“If they try to travel this road by themselves, it will be perilous,” says Bernard Munos, a researcher and senior fellow who focuses on biomedical innovation at the Milken Institute, a conservative think tank.
“It’s like sending a man to the moon. On paper it may look fairly straightforward, but the details are what will cause the enterprise to succeed or fail.”
Before the company’s second post-SPAC earnings report, on Nov. 10, Wojcicki says she’s still working out what the integration of Lemonaid will look like. For starters, 23andMe will train Lemonaid’s fleet of doctors on how to make use of genetic information and try to keep its services cheap enough that insurance doesn’t have to get involved.
(Lemonaid’s services, like consumer DNA kits, aren’t covered.) “When I think back on the original promise of genetics, it’s been adopted in cancer treatment but not at all in primary care,” she says, adding that the company is trying to figure out what actionable health recommendations based on genetic risk factors should be.
In the early going, that will most likely mean using its pharmacogenetics reports—which help determine which drugs are likely to work best for an individual—when prescribing drugs through Lemonaid doctors.
Your genes can affect how well you metabolize certain drugs and may influence, for example, which antidepressant or antifungal works best with your biology.
Eventually, Wojcicki says, that might lead 23andMe to be involved in customers’ health care in two ways: using the consumer testing to head off illness while the drugmaking side manages those that can’t be prevented. “In five years,” she says, “what I am looking to be measured on is, can we keep people healthier?”
As for whether these new business lines might exacerbate customers’ privacy concerns, Wojcicki’s main counterargument is that the U.S. medical establishment is lousy on privacy, too.
For years the buying and selling of health data has expanded dramatically without much doctors or patients can do about it, if they’re even aware. Unlike when you go to the doctor, she says, 23andMe customers have a choice about how their data are used.
Well, kind of. There are no federal laws prohibiting companies outside of a health-care setting from providing individuals’ genetic information to third parties, and the existing protections of genetic data in the U.S. are weak at best.
That became clear in 2018, when police used a different, open source database called GEDmatch to make an arrest in the long-cold Golden State Killer murders.
Suddenly consumers everywhere were very aware of just how serious the consequences of sharing your DNA can be, which apparently made them less enthusiastic about home DNA kits. 23andMe’s sales dropped off, and layoffs followed in early 2020.
While calls to strengthen consumer DNA protections died down during the pandemic, 23andMe’s latest development may help to reignite those efforts.
“They’re transparent, but only to a certain degree,” says Jennifer King, a privacy and data policy fellow at Stanford’s Center for Internet and Society. “My data could be extremely valuable to them.” King says a better system would require a third party to broker data and make sure consumers are compensated fairly.
In some cases, after all, one individual can hold the key to a world of biomedicine. Take the famous case of Henrietta Lacks, whose family struggled in poverty for years after researchers turned her cancer cells into a critical research tool that made millions of dollars.
With a far greater range of the human genome decoded, it’s easy to envision a Gattaca-esque future in which the DNA of the masses is mined for personalized miracle cures affordable only to the super rich.
Wojcicki says that’s just not going to happen. “We’re not evil,” she says. “Our brand is being direct-to-consumer and affordable.” For the time being she’s focused on the long, painful process of drug development. She’d like to think she’s earned some trust, but she hasn’t come this far on faith.
Updated: 11-29-2021
Princeton Team Disables Long-Targeted Gene Behind Spread Of Major Cancers
The mysterious ways cancer spreads through the body, a process known as metastasis, is what can make it such a difficult enemy to keep at bay. Researchers at Princeton University working in this area have been tugging at a particular thread for more than 15 years, focusing on a single gene central to the ability of most major cancers to metastasize.
They’ve now discovered what they describe as a “silver bullet” in the form of a compound that can disable this gene in mice and human tissue, with clinical trials possibly not too far away.
Metastatic cancer is a key focus for researchers and with good reason, as it is actually the primary cause of death from the disease. While surgery or chemotherapy might be effective at eliminating an initial tumor, cells that have broken away can discreetly make their way around the body and give rise to new tumors, months or even years later.
“Metastatic breast cancer causes more than 40,000 deaths every year in the US, and the patients do not respond well to standard treatments, such as chemotherapies, targeted therapies and immunotherapies,” says Minhong Shen, member of the Princeton team behind the new discovery.
“Our work identified a series of chemical compounds that could significantly enhance the chemotherapy and immunotherapy response rates in metastatic breast cancer mouse models. These compounds have great therapeutic potential.”
This discovery has its roots in 2004 research in which Princeton scientists identified a gene implicated in metastatic breast cancer, called metadherin, or MTDH. A 2009 paper by cancer biologist Yibin Kang then showed the gene was amplified and produced abnormally high levels of MTDH proteins in around a third of breast cancer tumors, and was central to not just the process of metastasis, but also the resistance of those tumors to chemotherapy.
“There was a lot of excitement,” Kang says. “‘Wow, we found a metastasis gene related to poor outcomes in patients! What next? Can we target it?’ That was the big question, because at the time, nobody knew how this obscure, little-known gene worked. It had no similarity to any other known human protein. We didn’t know if it was important to normal physiology.”
Subsequent research continued to shed light on the importance of the MTDH gene, demonstrating how it is critical for cancer to flourish and metastasize. Mice engineered to lack the gene grew normally, and those that did get breast cancer featured far fewer tumors – and those tumors that did form didn’t metastasize.
This was then found to be true of prostate cancer, lung cancer, colorectal cancer, liver cancer and many other cancers.
“So basically, in most major human cancers, this gene is essential for cancer progression and all the terrible things associated with cancer, and yet it doesn’t seem to be important for normal development,” says Kang. “Mice can grow and breed and live normally without this gene, so we knew this would be a great drug target.”
The crystal structure of MTDH shows the protein has a pair of protrusions likened to fingers, which interlock with two holes in the surface of another protein called SND1. This is “like two fingers sticking into the holes of a bowling ball,” according to Kang, and the scientists suspected if this intimate connection could be broken, it could go a long way to dampening the harmful effects of MTDH.
“We knew from the crystal structure what the shape of the keyhole was, so we kept looking until we found the key,” Kang says.
The team spent two years screening for the right molecules to fill these holes without any great success, until they landed on what they say is a “silver bullet.”
The resulting compound plugs these voids and prevents the proteins from interlocking, with profound anti-cancer effects that resemble those seen in the MTDH-deficient mice from their earlier work.
“In 2014, we showed what happens if you knock out a gene at birth,” Kang says. “This time, we show that after the tumor has already fully developed into full-blown, life-threatening cancer, we can eliminate the function of this gene. We found that whether you do it genetically or pharmacologically using our compound, you achieve the same outcome.”
The scientists say that MTDH assists cancer in two primary ways, by helping tumors endure the stresses of chemotherapy and by silencing the alarm that organs normally sound when a tumor invades them.
By interlocking with the SND1 protein, it prevents the immune system from recognizing the danger signals normally generated by cancerous cells, and therefore stops it from attacking them.
“Now, with this drug, we reactivate the alarm system,” Kang says. “In normal tissues, healthy cells are usually not under stress or presenting signals that can be recognized as foreign by the immune system, so this is why MTDH is not essential for normal tissues. In essence, MTDH is a quintessential ‘cancer fitness gene’ that is uniquely required by malignant cells to survive and thrive.”
The team is now working to refine the compound, hoping to improve its effectiveness in disrupting the connection between MTDH and SND1 and lower the required dosage. While noting that they are only just scratching the surface in terms of what it could do, they hope to be ready for clinical trials on human patients in two to three years.
“In the two papers we are publishing back-to-back today, we identify a compound, show it is effective against cancer, and show that it is very, very effective when combined with chemotherapy and immunotherapy,” says Kang. “Even though metastatic cancers are scary, by figuring out how they work – figuring out their dependency on certain key pathways like MTDH – we can attack them and make them susceptible to treatment.”
The research was published across two papers in the journal Nature Cancer .
Updated: 4-19-2022
Bacteria Linked To Prostate Cancer Raises Hope Of Breakthrough
Researchers have identified five types of bacteria that are linked to aggressive prostate cancer.
The bacteria was common in urine and tissue samples from men with the condition, a new study found.
It is hoped the findings could help pave the way for treatments that could target this bacteria and slow or prevent the development of aggressive disease.
Scientists do not yet know how people pick up the bacteria, or whether they are causing the disease.
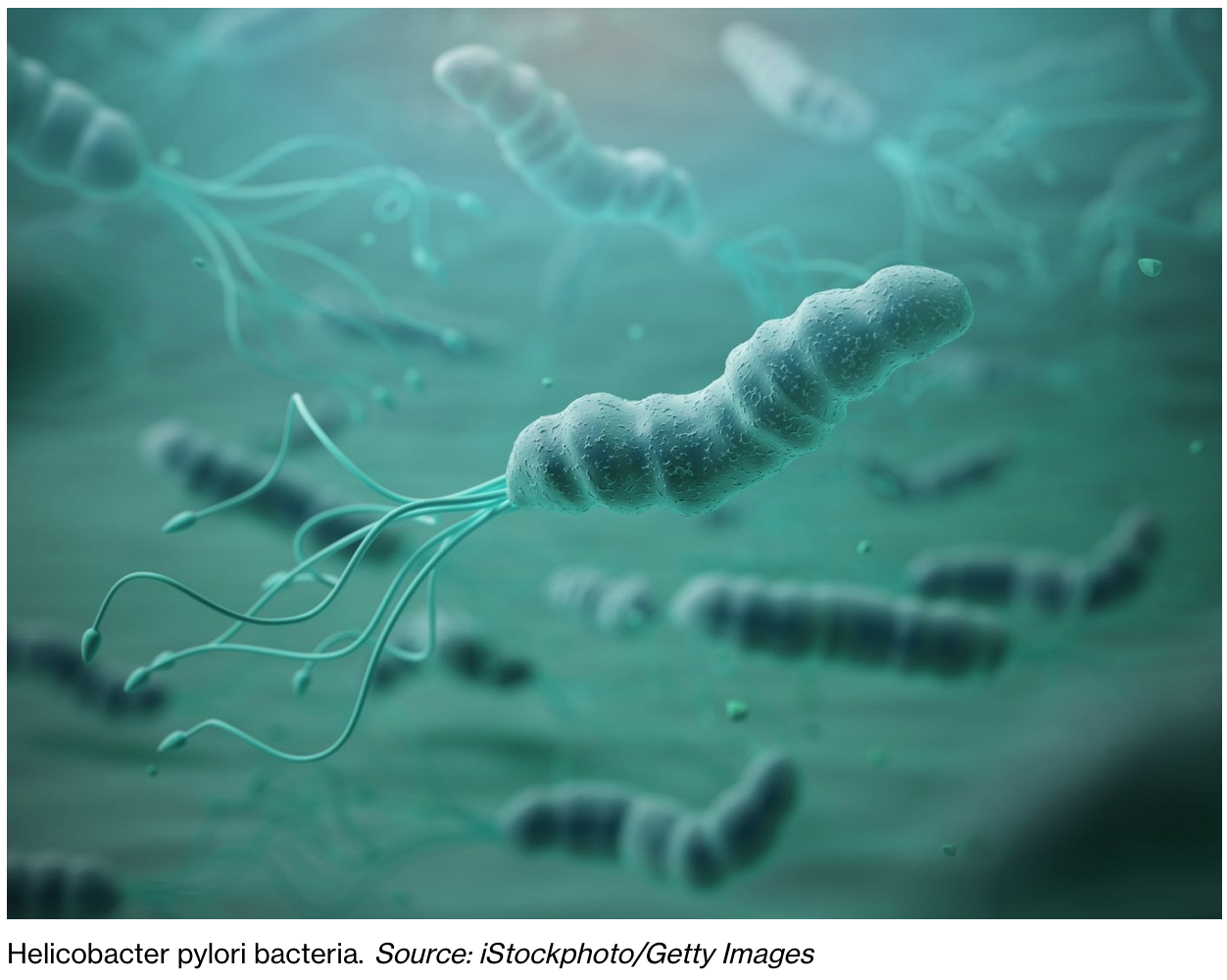
Project lead Professor Colin Cooper from the University of East Anglia’s (UEA) Norwich Medical School, said: “We already know of some strong associations between infections and cancer.
“For example, the presence of Helicobacter pylori bacteria in the digestive tract can lead to stomach ulcers and is associated with stomach cancer, and some types of the HPV virus can cause cervical cancer.”
“We wanted to find out whether bacteria could be linked to the way prostate cancer grows and spreads.”
Dr Jeremy Clark, also from UEA’s Norwich Medical School, said: “While prostate cancer is responsible for a large proportion of all male cancer deaths, it is more commonly a disease men die with rather than from.”
“And little is known about what causes some prostate cancers to become more aggressive than others.”
“We now have evidence that certain bacteria are involved in this and are part of the puzzle.”
The team worked with researchers at the Norfolk and Norwich University Hospital, the Quadram Institute, and other collaborators to analyse urine or tissue samples from more than 600 patients with or without prostate cancer.
They developed methods of finding the bacteria associated with aggressive prostate cancer.
Dr Rachel Hurst, first author of this work and also from UEA’s Norwich Medical School, said: “We found several types of bacteria associated with aggressive prostate cancer, some of which are new types of bacteria never found before.”
Two of the new bacteria species found by the team have been named after two of the study’s funders – Porphyromonas bobii, after the The Bob Champion Cancer Trust, and Varibaculum prostatecancerukia, after Prostate Cancer UK.
The set of bacteria found by the team include Anaerococcus, Peptoniphilus, Porphyromonas, Fenollaria and Fusobacterium.
All of the bacteria like to grow without oxygen present.
Dr Hurst said: “When any of these specific anaerobic bacteria were detected in the patient’s samples, it was linked to the presence of higher grades of prostate cancer and more rapid progression to aggressive disease.”
“We also identified potential biological mechanisms of how these bacteria may be linked to cancer.”
“Among the things we don’t yet know is how people pick up these bacteria, whether they are causing the cancer, or whether a poor immune response permits the growth of the bacteria.”
“But we hope that our findings and future work could lead to new treatment options that could slow or prevent aggressive prostate cancer from developing.”
“Our work could also lay the foundations for new tests that use bacteria to predict the most effective treatment for each man’s cancer.”
The researchers also noted many bacteria are beneficial to human life and it is not a simple matter to remove the harmful bacteria without removing the protection provided by the good bacteria.
The study, published in European Urology Oncology, was funded by The Bob Champion Cancer Trust and Prostate Cancer UK.
Updated: 6-6-2022
This Drug Could Transform Breast Cancer Treatment
The early success of Enhertu, from AstraZeneca and Daiichi Sankyo, bodes well for an entire class of medicines that may one day replace conventional chemotherapy.
New data on a breast cancer treatment from AstraZeneca and the Japanese drug maker Daiichi Sankyo brought a standing ovation from cancer doctors attending their annual meeting in Chicago on Sunday. And with good reason.
The drug, Enhertu, enabled women with advanced breast cancer to live six months longer than others treated with conventional chemotherapy. Oncologists call the results “practice changing” for metastatic breast cancer.
And the good news extends well beyond breast cancer. The evidence suggests the drug could one day be used to treat many other types of tumors — opening broad opportunities for Enhertu and other drugs like it.
Enhertu is what’s known as an antibody-drug conjugate, because it uses an antibody to home in on a tumor cell before releasing a payload of toxic chemotherapy to kill it. This drug’s antibody zeroes in on a protein called HER2, a growth signal that in some breast cancers becomes too amped up.
The US Food and Drug Administration approved Enhertu in late 2019 for some so-called “HER2 positive” cancers, or tumors that on a diagnostic test show a certain threshold of that signal.
The new data are from breast cancers that have low, even negligible levels of HER2. That means a far wider range of patients might benefit from the drug — as many as 55% of breast cancers that were previously considered “HER2 negative” could actually have these low levels of the protein.
Other HER2-targeted drugs have been studied in patients whose tumors express low levels of the protein, but none have helped. Oncologists have a few theories about what makes Enhertu different.
One possibility is that there’s just enough HER2 being expressed on the cancer-cell surface to make it “sticky” enough for Enhertu’s antibody to attach and then deliver the powerful chemo.
Another idea is related to tumors’ inherent variability. “When you look at breast cancers under a microscope, not every cell is the same,” says Harold Burnstein, a breast cancer specialist at Dana Farber Cancer Institute, in Boston.
If enough cells with HER2 on the surface can lure the drug to the tumor, the chemo can also pick off nearby cancer cells that lack the key protein.
The design of the drug itself could also matter. Daiichi Sankyo, which discovered the drug before striking a deal to develop it with AstraZeneca in 2019, seems to have ironed out many of the kinks that limited the success of earlier antibody-drug conjugates.
Enhertu uses a different, more powerful chemo payload and a bigger one, too. Whereas most antibody-drug conjugates carry two to four chemo molecules, Enhertu delivers eight. The payload’s effect is also more fleeting, to help minimize side effects from the very toxic chemo.
This suggests that, after decades of trial and error with antibody-drug conjugates, cancer researchers are finally starting to understand how best to design and use them.
And the success of this trial could mean the drug will be used well beyond breast cancer. Many other kinds of tumors express low levels of HER2, including gastric, colon and lung cancer. AstraZeneca is now studying Enhertu in these cancers. “The upside of this drug is yet to be fully realized,” Burnstein says.
The evidence adds to a growing body of research suggesting that antibody-drug conjugates will one day replace conventional chemo for most patients, says Maryam Lustberg, chief of Breast Medical Oncology at Yale Cancer Center.
The drugs are not without downsides. Enhertu’s design allows an otherwise too-toxic chemotherapy to be used, but the side effects of the drug — and antibody-drug conjugates in general — are similar to those of all chemo.
And these drugs are much more expensive, potentially putting them out of reach for the uninsured or for breast cancer patients in low-income countries.
Nevertheless, Enhertu is teaching the field how to design drugs in this class to maximize their benefit. That could mean effective treatment for a wide universe of patients.
Updated: 6-30-2022
The Quest To Understand Skin Cancer
The 20th-century surgeon Frederic Mohs made a key breakthrough in treating a disease first described in ancient Greece.
July 1 marks the 20th anniversary of the death of Dr. Frederic Mohs, the Wisconsin surgeon who revolutionized the treatment of skin cancer, the most common form of cancer in the U.S. Before Mohs achieved his breakthrough in 1936, the best available treatment was drastic surgery without even the certainty of a cure.
Skin cancer is by no means a new illness or confined to one part of the world; paleopathologists have found evidence of it in the skeletons of 2,400- year-old Peruvian mummies. But it wasn’t recognized as a distinct cancer by ancient physicians.
Hippocrates in the 5th century B.C. came the closest, noting the existence of deadly “black tumors (melas oma) with metastasis.” He was almost certainly describing malignant melanoma, a skin cancer that spreads quickly, as opposed to the other two main types, basal cell and squamous cell carcinoma.
The ‘fungoid disease,’ as some referred to skin cancer, yielded up its secrets by slow degrees.
After Hippocrates, nearly 2,000 years elapsed before earnest discussions about black metastasizing tumors began to appear in medical writings. The first surgical removal of a melanoma took place in London in 1787.
The surgeon involved, a Scotsman named John Hunter, was mystified by the large squishy thing he had removed from his patient’s jaw, calling it a “cancerous fungus excrescence.”
The “fungoid disease,” as some referred to skin cancer, yielded up its secrets by slow degrees. In 1806 René Laënnec, the inventor of the stethoscope, published a paper in France on the metastatic properties of “La Melanose.”
Two decades later, Arthur Jacob in Ireland identified basal cell carcinoma, which was initially referred to as “rodent ulcer” because the ragged edges of the tumors looked as though they had been gnawed by a mouse.
By the beginning of the 20th century, doctors had become increasingly adept at identifying skin cancers in animals as well as humans, making the lack of treatment options all the more frustrating.
In 1933, Mohs was a 23-year-old medical student assisting on cancer research in rats when he noticed the destructive effect of zinc chloride on malignant tissue.
Excited by its potential, within three years he had developed a zinc chloride paste and a technique for using it on cancerous lesions.
He initially described it as “chemosurgery” since the cancer was removed layer by layer. The results for his patients, all of whom were either inmates of the local prison or the mental health hospital, were astounding.
Even so, his method was so novel that the Dane County Medical Association in Wisconsin accused him of quackery and tried to revoke his medical license.
Mohs continued to encounter stiff resistance until the early 1940s, when the Quislings, a prominent Wisconsin family, turned to him out of sheer desperation. Their son, Abe, had a lemon-sized tumor on his neck which other doctors had declared to be inoperable and fatal.
His recovery silenced Mohs’s critics, although the doubters remained an obstacle for several more decades. Nowadays, a modern version of ”Mohs surgery,” using a scalpel instead of a paste, is the gold standard for treating many forms of skin cancer.
Nevertheless, an ounce of prevention is still worth a pound of cure. We have known since the 1950s that prolonged exposure to UV radiation causes skin cancer. This summer, when you go outside, enjoy the sun but wear a hat and above all, apply sunscreen.
Updated: 7-7-2022
‘Next-Gen’ Cancer Therapy Can Kill Tumours Without Harming Healthy Cells
Researchers in the US have developed a novel strategy to improve a cancer therapy that can kill tumor cells while leaving nearby healthy tissues intact.
Among the most promising anti-cancer treatments in recent years, oncolytic virotherapy (OV) has emerged at the top of the pack of immunotherapy. Oncolytic viruses can kill cancer cells while leaving nearby healthy cells and tissues intact.
In oncolytic virotherapy, the treatment also exerts its influence by activating an antitumor immune response made of immune cells such as natural killer (NK) cells.
However, sometimes those natural killers limit the oncolytic viruses, and so despite the exciting development in the OV field in recent years, there is room for improvement to tackle some limitations, including the relatively weak therapeutic activity and lack of means for effective systemic delivery.
Those improvements are now being made in the lab of Shaun Zhang, director of the Center for Nuclear Receptors and Cell Signaling at the University of Houston, US, who has received a USD 1.8 million grant from the National Institutes of Health to support his work.
”We have developed a novel strategy that not only can prevent NK cells from clearing the administered oncolytic virus, but also goes one step further by guiding them to attack tumor cells,” Zhang said.
”We took an entirely different approach to create this oncolytic virotherapy by deleting a region of the gene which has been shown to activate the signaling pathway that enables the virus to replicate in normal cells,” he said.
The different approach consists of a new oncolytic virus called FusOn-H2, based on the Herpes simplex 2 virus, (HSV-2), commonly known as genital herpes.
They are the virus with an NK cell engager, resulting in what Zhang calls the ”two birds with one stone strategy” to enhance the therapeutic effect of the new oncolytic virus.
This engager forms a bridge between NK cells and tumor cells, resulting in the killing of the engaged tumor cells, the researchers said.
”Our recent studies showed that arming FusOn-H2 with a chimeric NK engager that can engage the infiltrated natural killer cells with tumor cells could significantly enhance the effectiveness of this virotherapy,” said Zhang.
”Most importantly, we observed that tumor destruction by the joint effect of the direct oncolysis and the engaged NK cells led to a measurable elicitation of neoantigen-specific antitumor immunity,” he added.
Zhang and the team believe that this armed FusOn-H2 will produce a three-pronged effect to enhance the antitumor efficacy against solid tumors in colon and lung cancer, which they expect to come in waves.
The first wave comes immediately after the armed virus is administered and it derives primarily from the administration of the virus, according to the researchers.
The second wave comes from the natural killer cells doing their work while the third wave is the outcome of a series of chain events that ultimately result in inducing neoantigen-specific antitumor immunity, they said.
”We hypothesize that the combination of the high potency of the three-pronged therapy with the improved systemic delivery will lead to effective treatment of metastatic diseases,” Zhang added.
Updated: 12-11-2022
Base Editing: Revolutionary Therapy Clears Girl’s Incurable Cancer
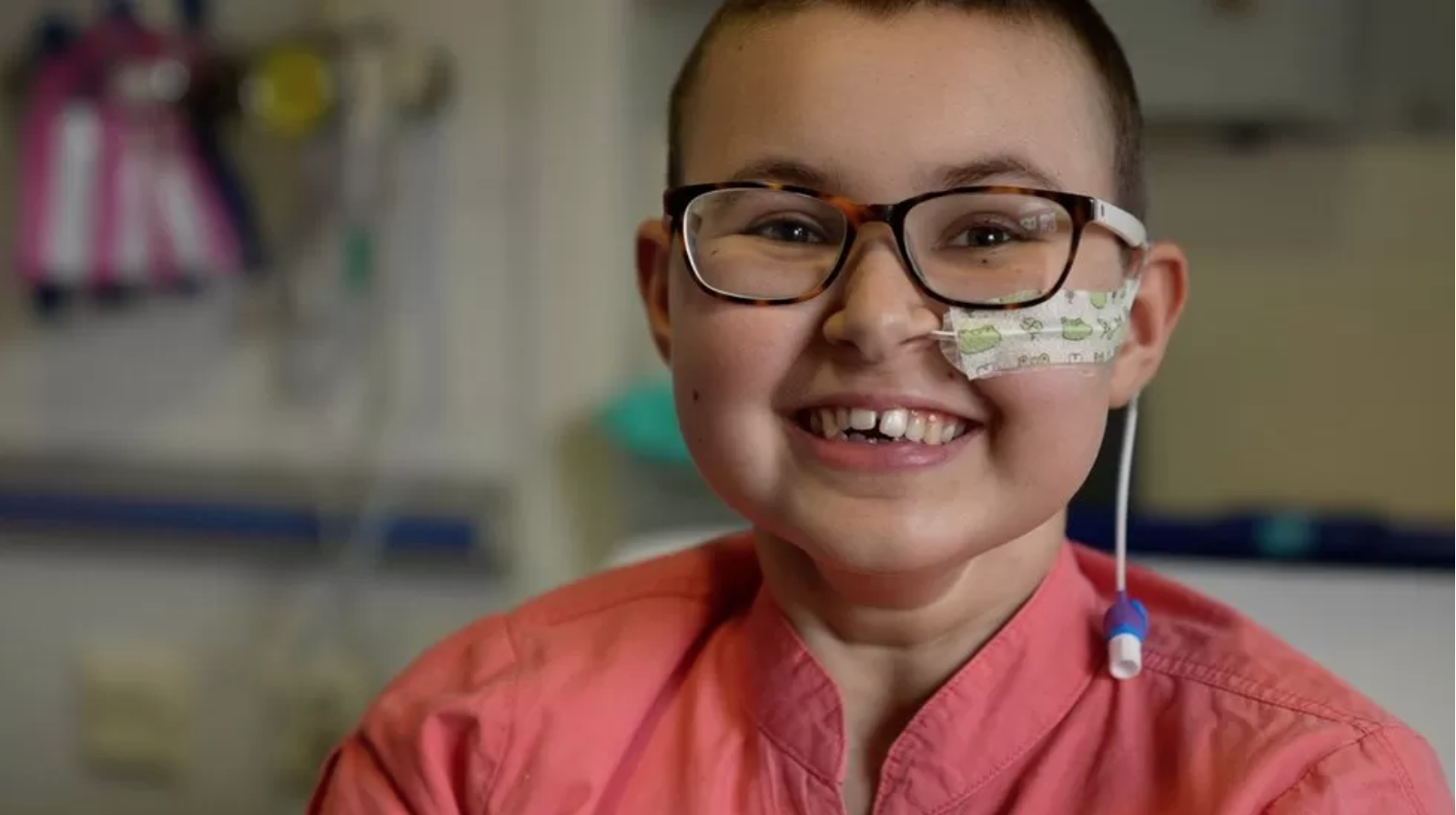
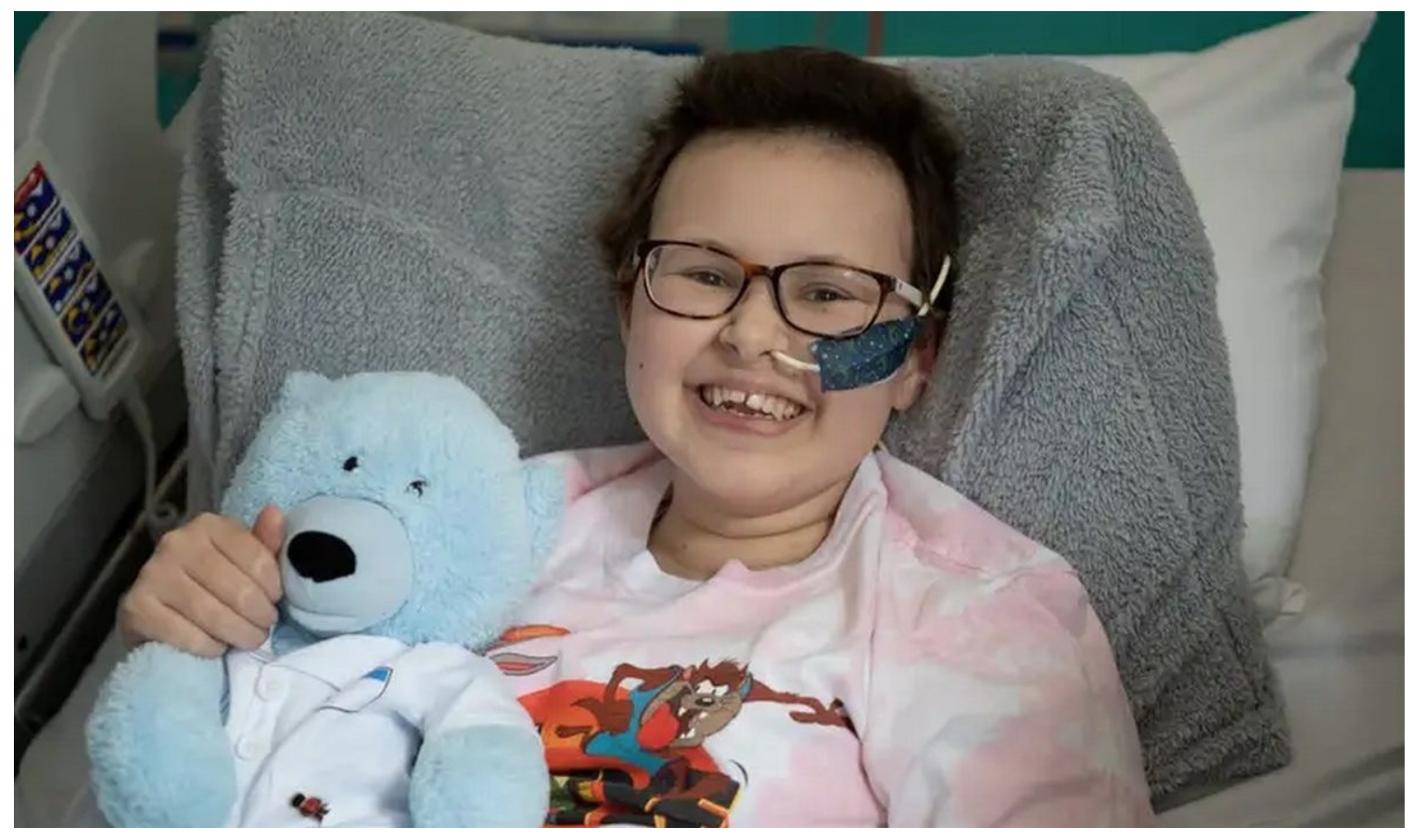
A teenage girl’s incurable cancer has been cleared from her body in the first use of a revolutionary new type of medicine.
All other treatments for Alyssa’s leukaemia had failed.
So doctors at Great Ormond Street Hospital used “base editing” to perform a feat of biological engineering to build her a new living drug.
Six months later the cancer is undetectable, but Alyssa is still being monitored in case it comes back.
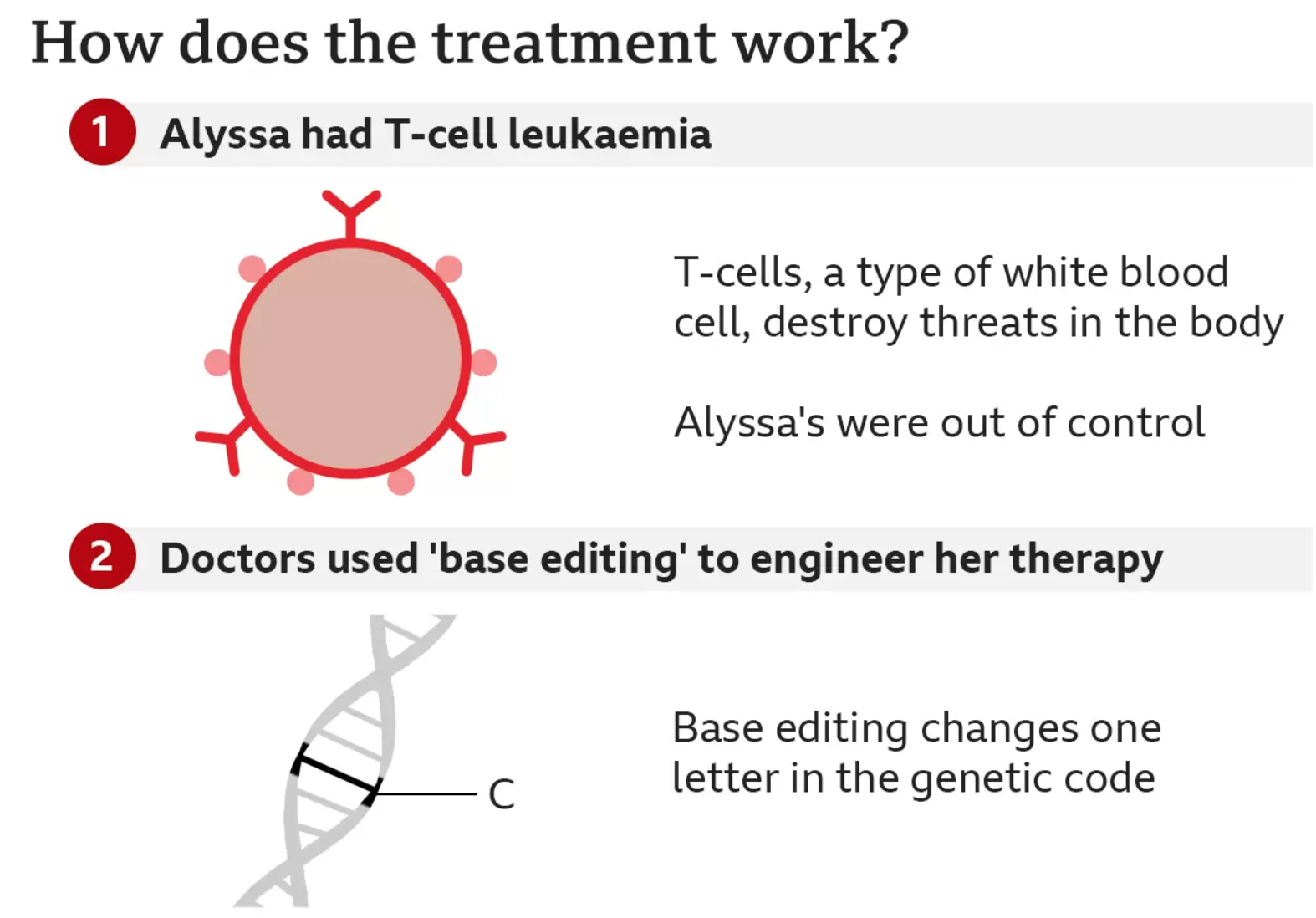
Alyssa, who is 13 and from Leicester, was diagnosed with T-cell acute lymphoblastic leukaemia in May last year.
T-cells are supposed to be the body’s guardians – seeking out and destroying threats – but for Alyssa they had become the danger and were growing out of control.
Her cancer was aggressive. Chemotherapy, and then a bone-marrow transplant, were unable to rid it from her body.
Without the experimental medicine, the only option left would have been merely to make Alyssa as comfortable as possible.
“Eventually I would have passed away,” said Alyssa. Her mum, Kiona, said this time last year she had been dreading Christmas, “thinking this is our last with her”. And then she “just cried” through her daughter’s 13th birthday in January.
What happened next would have been unthinkable just a few years ago and has been made possible by incredible advances in genetics.
The team at Great Ormond Street used a technology called base editing, which was invented only six years ago.
Bases are the language of life. The four types of base – adenine (A), cytosine (C), guanine (G) and thymine (T) – are the building blocks of our genetic code. Just as letters in the alphabet spell out words that carry meaning, the billions of bases in our DNA spell out the instruction manual for our body.
Base editing allows scientists to zoom to a precise part of the genetic code and then alter the molecular structure of just one base, converting it into another and changing the genetic instructions.
The large team of doctors and scientists used this tool to engineer a new type of T-cell that was capable of hunting down and killing Alyssa’s cancerous T-cells.
They started with healthy T-cells that came from a donor and set about modifying them.
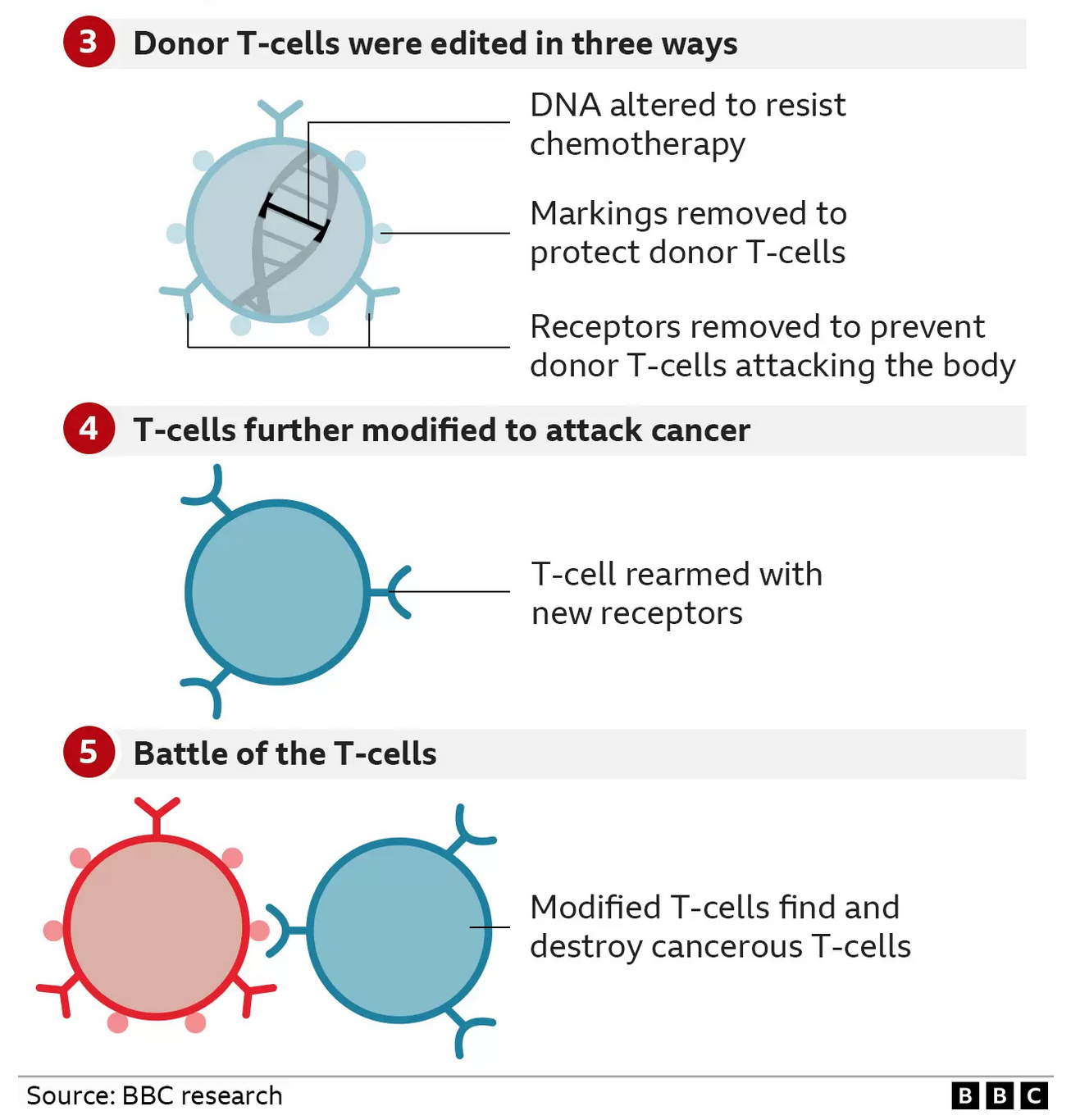
* The first base edit disabled the T-cells targeting mechanism so they would not assault Alyssa’s body
* The second removed a chemical marking, called CD7, which is on all T-cells
* The third edit was an invisibility cloak that prevented the cells being killed by a chemotherapy drug
The final stage of genetic modification instructed the T-cells to go hunting for anything with the CD7 marking on it so that it would destroy every T-cell in her body – including the cancerous ones. That’s why this marking has to be removed from the therapy – otherwise it would just destroy itself.
If the therapy works, Alyssa’s immune system – including T-cells – will be rebuilt with the second bone-marrow transplant.
When the idea was explained to the family, mum Kiona was left thinking: “You can do that?” It was Alyssa’s decision to be the first to take the experimental therapy – which contained millions of the modified cells – in May this year.
“She’s the first patient to be treated with this technology,” said Prof Waseem Qasim, from UCL and Great Ormond Street.
He said this genetic manipulation was a “very fast-moving area of science” with “enormous potential” across a range of diseases.
Alyssa was left vulnerable to infection, as the designer cells attacked both the cancerous T-cells in her body and those that protect her from disease.
After a month, Alyssa was in remission and was given a second bone-marrow transplant to regrow her immune system.
Alyssa spent 16 weeks in hospital and couldn’t see her brother, who was still going to school, in case he brought germs in.
There were worries after the three-month check-up found signs of the cancer again. But her two most recent investigations have been clear.
“You just learn to appreciate every little thing. I’m just so grateful that I’m here now,” said Alyssa.
“It’s crazy. It’s just amazing I’ve been able to have this opportunity, I’m very thankful for it and it’s going to help other children, as well, in the future.”
She’s eyeing-up Christmas, being a bridesmaid at her auntie’s wedding, getting back on her bike, going back to school and “just doing normal people stuff”.
The family hope the cancer will never return, but are already grateful for the time it has bought them.
“To have this extra year, this last three months when she’s been home, has been a gift in itself,” said Kiona.
Dad James said: “I find it quite hard to talk about how proud we are. When you see what she’s gone through and her vitality of life she’s brought to every situation, it’s outstanding.”
Most children with a leukaemia respond to the main treatments, but it is thought that up to a dozen a year could benefit from this therapy.
Alyssa is just the first of 10 people to be given the drug as part of a clinical trial.
Dr Robert Chiesa, from the bone-marrow transplant department at Great Ormond Street Hospital, said: “It is extremely exciting. Obviously, this is a new field in medicine and it’s fascinating that we can redirect the immune system to fight cancer.”
The technology, though, only scratches the surface of what base editing could achieve.
Dr David Liu, one of the inventors of base editing at the Broad Institute, told me it was “a bit surreal” that people were being treated just six years after the technology was invented.
In Alyssa’s therapy, each of the base edits involved breaking a section of genetic code so it no longer worked. But there are more nuanced applications where instead of switching an instruction off you can fix a defective one. Sickle-cell anaemia, for example, is caused by just one base change that could be corrected.
So there are already trials of base editing under way in sickle-cell disease, as well as high cholesterol that runs in families and the blood disorder beta-thalassemia.
Dr Liu said the “therapeutic applications of base editing are just beginning” and it was “humbling to be part of this era of therapeutic human gene editing”, as science was now taking “key steps towards taking control of our genomes”.
Updated: 12-20-2022
Aggressive Leukemia Disappears In 13-Year-Old Girl Who Was First To Receive New CRISPR Treatment
In the latest CRISPR success story, a 13-year-old girl whose leukemia had not responded to other treatments now has no detectable cancer cells.
She received a dose of immune cells that were genetically edited to attack the leukemia, a method that’s been used with other cancers.
A form of cancer in the bone marrow tissue, leukemia is caused by mutated immune cells and is normally treated by killing all bone marrow cells in the patient’s body before receiving a transplant from a donor. If this falls, the Nobel Prize-winning CAR-T cell therapy can be used instead.
This was the case of Alyssa, a 13-year-old girl from the UK, who received a dose of common immune system weapons called T cells that had been modified to attack cancerous cells in her body. To avoid the extreme costs associated with this, the Great Ormond Street Hospital team at University College London further modified the donor T cells.
“This is quite remarkable, although it is still a preliminary result, which needs to be monitored and confirmed over the next few months,” said Robert Chiesa, one of the doctors treating Alyssa, in a statement released by Great Ormond Street Hospital.
While she has no detectable cancer now, it will take several years to determine whether she’s truly cancer-free.
This procedure was used before to save the life of a 1-year-old girl, Layla, also in the UK last year, and is now approved by the NHS as a treatment for people with leukemia arising from mutated B cells, another group of immune cells that can lead to the cancer.
New Scientist explains that lead researcher Waseem Qasim at University College London had applied four edits to the cells’ DNA, which puts them at risk for dangerous mutations. To circumvent this, he used a different method of the CRISPR gene editing protein that ” changes one DNA letter to another,” a technique called base-editing.
Alyssa is the first person ever to be treated with base-editing.
Related Article:
Which Lipsticks Contain The Most Lead, Cadmium, Aluminum And Other Heavy Metals? (#GotBitcoin?)
Can An Extra $333 A Month Improve A Baby’s Brain? A Research Team Wants To Know (#GotBitcoin?)
Why Women Live Longer Than Men (#GotBitcoin?)
Why Men Won’t Go To The Doctor, And How To Change That (#GotBitcoin?)
Ultimate Guide To Financial Resources For Cancer Patients
He Conquered Cancer, Then 15 Spartan Races In A Year (#GotBitcoin?)
Fighting Cancer By Releasing The Brakes On The Immune System (#GotBitcoin?)
Over-Diagnosis And Over-Treatment Of Cancer In America Reaches Crisis Levels (#GotBitcoin?)
Cancer Super-Survivors Use Their Own Bodies To Fight The Disease (#GotBitcoin?)
Your Questions And Comments Are Greatly Appreciated.
Monty H. & Carolyn A.
Go back










Leave a Reply
You must be logged in to post a comment.